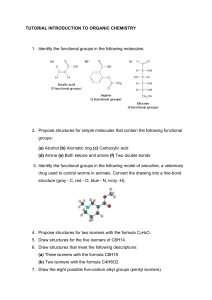
1. The molecular formula C3H6Br2 is that of 2,2-Dibromopropane but it could also be that of other organic molecules. What type of isomerism is being described in this situation? Draw all possible isomers of this compound. 2. Explain how an enantiomer and a diastereomer differ from each other. Give an example of an everyday object that might help you better illustrate the difference between these two types of isomers. Stereoisomers are broken into two categories, enantiomers and diastereomers. Stereoisomer compounds with molecules that are not mirrored images of one another and also not superimposable are Diastereomers. They have different physical and chemical properties. An example of this is sugars and amino acids. Chiral molecule that are mirror images of one another and also superimposable are Enantiomers. They have identical physical and chemical properties and bend light in opposite directions. An example of this your hands they are very similar to each other but there not the same. 3.Write structural formulas and the IUPAC names for three constitutionals (i.e. structural) isomers with the molecular formula C9H20. (3 points) 4.Write the structural formulas for the two geometric isomers of 2,3dibromomethylhex-2-ene. Write the names for both isomers. 5. Give systematic IUPAC names for each of the following: (0.5 points each) 6. Write structural formulas of isomers with the same molecular formula of C5H12O for: (1 point each) a) one primary alcohol - 1-Pentanol: CH3(CH2)3CH2OH b) one secondary alcohol- 2-Pentanol: CH3(CH2)2CHOHCH3 c) one tertiary alcohol- 2-Methyl-2-butanol: 7. Explain the difference between a secondary amide and a secondary amine. Your explanation must contain an example of each. Amide and Amine are nitrogenous compounds however their structures and properties differ. Secondary amines have two organic substituents bound to the nitrogen together with one hydrogen. An example of a secondary amine is dimethylamine. Secondary amides are amides in which the nitrogen atom is directly bonded to two carbon atoms, the carbonyl group plus one other carbon. An example of a secondary amide is benzamide. 8. Draw both the condensed (ie, CH3CH2CH3) and the structural formula (hand drawn) for each of the following compounds: (2 points each) a) 2,2-dimethyl-3-ethylpentane b) 2,3-butandiol c) trans-5-methyl-2-heptene d) 6-chloro-2-methyl-3-heptyne e) 1,2-dicholorobenzene 9.Aspartame is an artificial sweetener that became increasingly common in the 1990's. Below you will find the structure of aspartame. Circle all of the functional groups found on this molecule, and clearly name each functional group. (5 points)