Secondary One Science Worksheet: Mixtures, Compounds, Elements
advertisement
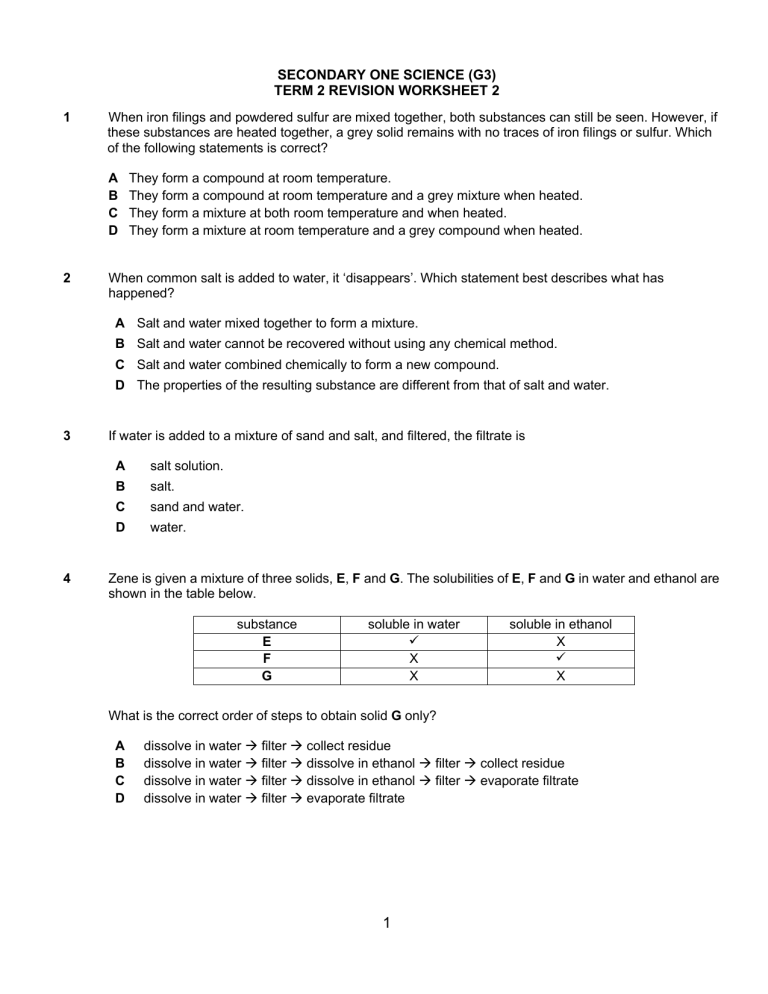
SECONDARY ONE SCIENCE (G3) TERM 2 REVISION WORKSHEET 2 1 When iron filings and powdered sulfur are mixed together, both substances can still be seen. However, if these substances are heated together, a grey solid remains with no traces of iron filings or sulfur. Which of the following statements is correct? A B C D 2 They form a compound at room temperature. They form a compound at room temperature and a grey mixture when heated. They form a mixture at both room temperature and when heated. They form a mixture at room temperature and a grey compound when heated. When common salt is added to water, it ‘disappears’. Which statement best describes what has happened? A Salt and water mixed together to form a mixture. B Salt and water cannot be recovered without using any chemical method. C Salt and water combined chemically to form a new compound. D The properties of the resulting substance are different from that of salt and water. 3 4 If water is added to a mixture of sand and salt, and filtered, the filtrate is A salt solution. B salt. C sand and water. D water. Zene is given a mixture of three solids, E, F and G. The solubilities of E, F and G in water and ethanol are shown in the table below. substance E F G soluble in water ✓ X X soluble in ethanol X ✓ X What is the correct order of steps to obtain solid G only? A B C D dissolve in water → filter → collect residue dissolve in water → filter → dissolve in ethanol → filter → collect residue dissolve in water → filter → dissolve in ethanol → filter → evaporate filtrate dissolve in water → filter → evaporate filtrate 1 5 6 Which of the following shows the correct classification of substances? compound mixture A pure water iron B carbon dioxide seawater C orange juice air D sodium chloride pure water Which of the following correctly describes the method used to differentiate a metal from a non- metal? A B C D 7 Which of the following is not an element? A B C D 8 observing the colour of the substances observing the physical state (i.e solid, liquid or gas) at room temperature testing for the electrical conductivity testing for the hardness of the substances calcium helium salt sulfur Which one of the following is a pure compound? A bronze B salt C seawater D steel Short-answer questions 9 The following are descriptions of three different substances, P, Q, R and S. P: It is a white solid. It can be separated into two substances by adding water and filtering. Q: It is a red solid with a constant composition. It decomposes into two elements when heated. R: It is a white solid. It is formed by burning magnesium in oxygen. S: (a) It is a gas. It does not decompose nor react when heated. Classify each of the substances as either an element, a compound or a mixture byplacing a tick (√) in the correct box. 2 substance element compound mixture P Q R S (b) [2] State two differences between compounds and mixture (check your textbook). …………………………………………………………………………………………………… …………………………………………………………………………………………………… …………………………………………………………………………………………………… 10 [2] The diagram below represents part of the Periodic Table. Use the codes P, Q, R, S and T to answer the following: (a) Write down the elements that are poor conductors of heat and electricity. ………………………………………………………...…………………………………………... (b) Write down the elements in period 3. ………………………………………………………...…………………………………………... (c) [1] [1] Using the Periodic Table, name the elements R and S. ………………………………………………………...…………………………………………... 3 [1] 11 Study the diagrams below. J K L K J M State which of the above diagrams, J, K, L and M, represent(s). Answers may be repeated. (a) three types of substances; ………………………… [1] (b) a mixture; ………………………… [1] (c) a compound; and ………………………… [1] (d) two elements. ………………………… [1] END OF WORKSHEET 4




