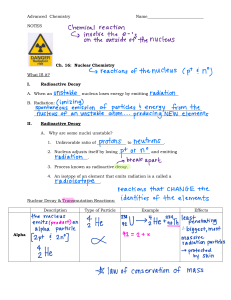
NUCLEAR CHEMISTRY 1.2.1 Radioactivity and Nuclear Reactions 1.2.2 Nuclear Stability 1.2.3 Energetics of Nuclear Reactions 1.2.4 Interaction of Radiation and Matter Chapter 2 A chemical reaction occur as result of the interaction between valence electrons around an atom’s nucleus. In 1896, Henri Becquerel, discovered radioactivity of chemical elements, thus calling it as radiochemistry. He expanded the field of chemistry to include nuclear changes when he discovered that uranium emitted radiation. After Becquerel’s discovery, radioactivity was popularized by Marie Sklodowska Curie, together with Pierre Curie, began studying radioactivity and completed much of the pioneering work on nuclear changes. They found that there were two kinds of radioactive particles emitted from compounds. They called the electrically charge negative (-) kind Beta (ß) particles and positive (+) kind alpha (𝛼) particles. RADIOACTIVITY Radioactivity is the act of emitting radiation spontaneously. This is done by an atomic nucleus that, for some reason, is unstable; it "wants" to give up some energy in order to shift to a more stable configuration. Radioactivity is a physical, not a biological, phenomenon. Simply stated, the radioactivity of a sample can be measured by counting how many atoms are spontaneously decaying each second. This can be done with instruments designed to detect the particular type of radiation emitted with each "decay" or disintegration. The actual number of disintegrations per second may be quite large. Scientists have agreed upon common units to use as a form of shorthand. Thus, a curie (abbreviated "Ci" and named after Pierre and Marie Curie, the discoverers of radium[87]) is simply a shorthand way of writing "37,000,000,000 disintegrations per second," the rate of disintegration occurring in 1 gram of radium. The more modern International System of Measurements (SI) unit for the same type of measurement is the Becquerel (abbreviated "Bq" and named after Henri Becquerel, the discoverer of radioactivity), which is simply a shorthand for "1 disintegration per second." ISOTOPES Isotopes [ahy-suh-tohps] are atoms with the same number of protons, but differing numbers of neutrons. In other words, they have different atomic weights. Isotopes are different forms of a single element. There are 275 isotopes of the 81 stable elements. There are over 800 radioactive isotopes, some of which are natural and some synthetic. Every element on the periodic table has multiple isotope forms. The chemical properties of isotopes of a single element tend to be nearly identical. The exception would be the isotopes of hydrogen since the number of neutrons has such a significant effect on the size of the hydrogen nucleus. The physical properties of isotopes are different from each other since these properties often depend on mass. This difference may be used to separate isotopes of an element from each other by using fractional distillation and diffusion. NUCLEAR REACTION A nuclear reaction is considered to be the process in which two nuclear particles (two nuclei or a nucleus and a nucleon) interact to produce two or more nuclear particles or ˠ-rays (gamma rays). Thus, a nuclear reaction must cause a transformation of at least one nuclide to another. Sometimes if a nucleus interacts with another nucleus or particle without changing the nature of any nuclide, the process is referred to as nuclear scattering, rather than a nuclear reaction. Perhaps the most notable nuclear reactions are the nuclear fusion reactions of light elements that power the energy production of stars and the Sun. Natural nuclear reactions occur also in the interaction between cosmic rays and matter. Most chemical reactions involve an element’s outer electrons as they are shared, swapped and bumped. Nuclear reactions are different. All the action takes place inside the nucleus. There are two types of nuclear reactions. The first is the radioactive decay of bonds within the nucleus that emit radiation when broken. The second is the “billiard ball type of reaction where a nucleus or a nuclear particle (like proton) collides with another nucleus or nuclear particle. 1. RADIOACTIVE DECAY Radioactive decay occurs when an unstable atomic nucleus loses energy by emitting energy in the form of emitted particles or electromagnetic waves, called radiation. Some isotopes of a given element are more unstable than others, causing a nuclear reaction which releases energy to achieve a more stable nuclear configuration. Such isotopes are radioactive, and are referred to as “radioisotopes.” This process gives off energy in the form of ionizing particles or radiation. No collision with other atoms is needed, it just happens spontaneously. The original atom is called the parent nuclide, while the atom after emission is called the daughter nuclide. Example, carbon 14 (parent nuclide) emits radiation to become nitrogen 14 (daughter nuclide). While all radioactive isotopes go through this process, each one has its own decay rate. TYPES OF DECAY The three types of radiation have different levels of penetrating power. Penetrating power refers to the energy with which the radiation particles are ejected from the atom. The higher the energy, the more the particles or light produced by radioactive decay will penetrate a substance. ⮚ ALPHA DECAY An alpha particle (α\alpha) is made up of two protons and two neutrons bound together. This type of radiation has a positive charge (due to the presence of two protons). An alpha particle is sometimes represented using the chemical symbol He 2+, because it has the same structure as a helium atom missing its two electrons—hence the overall charge of +2. Their massive size (compared to beta particles, for instance) means alpha particles have very low penetration power. Penetration power describes how easily the particles can pass through another material. Since alpha particles have a low penetration power, the outside layer of the human skin, for example, can block these particles. Alpha particle/decay are commonly given off or emitted by larger radioactive isotopes, examples are uranium, thorium, actinium, radium and transuranium elements. Alpha decay occurs because the nucleus of a radioisotope has too many protons. A nucleus with too many protons causes repulsion between these like charges. To reduce this repulsion, the nucleus emits an α particle. Examples of this can be seen in the decay of americium (Am) to neptunium (Np). ⮚ BETA DECAY In radioactive nuclei with too many neutrons, a neutron can be converted into an electron, called beta particle. Beta particles (β) have a higher penetration power than alpha particles (they are able to pass through thicker materials such as paper). During beta decay, the number of neutrons in the atom decreases by one, and the number of protons increases by one. Effectively, a neutron was converted into a proton in the decaying nucleus, in the process releasing a beta particle. Since the number of protons before and after the decay is different, the atom has changed into a different element. As mentioned above, beta particles emitted as radiation are high energy, high speed electrons or positrons that came from the nucleus. When atoms like potassium 40 give off beta particles, there is a net charge of zero. This process happens in three different ways: ● Neutron-proton ratio increase, so a neutron (neutral) changes into a proton and release an electron. ● Neutron-proton ratio decrease, so a proton changes into a neutron and releases a positron (beta particle) ● Neutron-proton ratio in the nucleus decrease, so the nucleus captures an electron and changes a proton into a neutron. Beta particles have average ionizing and penetrating power, they are between the strength of alpha particles and gamma rays. Beta particles, particularly strontium-90, are used for treating eye and bone cancers, Positron or beta plus decay, can be useful as a tracer during positron emission tomography, as in a PET scan. ⮚ GAMMA DECAY Some decay reactions release energy in the form of electromagnetic waves called gamma rays. Gamma radiation (γ) is part of the electromagnetic spectrum, just like visible light. However, unlike visible light, humans cannot see gamma rays, because they have a much higher frequency and energy than visible light. Gamma radiation has no mass or charge. This type of radiation is able to penetrate most common substances, including metals. The only substances that can absorb this radiation are thick lead and concrete. Gamma decay reactions occur if the energy of the radioisotope’s nucleus is too high, and the resulting atomic number and atomic mass remain unchanged during the course of the reaction. When a radioactive element decays, different nuclear particles are given off. These radioactive particles can be separated by an electric (magnetic field) and detected in the laboratory. Alpha ( ) particles = positively (+) charged particles Beta (ß) particles = negatively (-) charged particles Gamma ( ) particles = high energy particles with zero charge NUCLEAR STABILITY The nucleus is made of neutrons and protons. What causes them to stick together? Why do the protons not repel each other? Nuclear Stability refers to the elements whose nucleus is stable, it means that the nuclear stability denotes the stability of the elements. Those atoms which have stable nucleus are known as nuclear stable atom. It also helps to identify the stability of an isotope. The two main factors that determine nuclear stability are the neutron/proton ratio and the total number of nucleons in the nucleus. ISOTOPE Isotope is an element that has the same atomic number but different atomic mass compared to the periodic table. Every element has a proton, neutron, and electron. The number of protons is equal to the atomic number, and the number of electrons is equal to the protons, unless it is an ion. To determine the number of neutrons in an element you subtract the atomic number from the atomic mass of the element. Atomic mass is represented as (AA) and atomic number is represented as (ZZ) and neutrons are represented as (NN). A=N+Z A=N+Z The principal factor for determining whether a nucleus is stable is the neutron to proton ratio. Elements with (Z<20Z<20) are lighter and these elements' nuclei have a ratio of 1:1 and prefer to have the same amount of protons and neutrons. EXAMPLE 1.11.1: CARBON ISOTOPES Carbon has three isotopes that scientists commonly used: C12C12, C13C13, C14C14. What is the number of neutron, protons, total nucleons and N:ZN:Z ratio for the C12C12 nuclide? SOLUTION For this specific isotope, there are 12 total nucleons (AA. From the periodic table, we can see that ZZ for carbon (any of the isotopes) is 6, therefore N=A-Z (from Equation 1): 12−6=6(1.2)(1.2)12−6=6 The N:P ratio therefore is 6:6 or a 1:1. In fact 99% of all carbon in the earth is this isotope. CONNECTION BETWEEN NUCLEAR STABILITY AND RADIOACTIVE DECAY The nuclei of radioisotopes are unstable. In an attempt to reach a more stable arrangement of its neutrons and protons, the unstable nucleus will spontaneously decay to form a different nucleus. If the number of neutrons changes in the process (number of protons remains), a different isotopes is formed and an element remains (e.g. neutron emission). If the number of protons changes (different atomic number) in the process, then an atom of a different element is formed. This decomposition of the nucleus is referred to as radioactive decay. During radioactive decay an unstable nucleus spontaneously and randomly decomposes to form a different nucleus (or a different energy state – gamma decay), giving off radiation in the form of atomic particles or high energy rays. This decay occurs at a constant, predictable rate that is referred to as half-life. A stable nucleus will not undergo this kind of decay and is thus nonradioactive. ENERGETICS OF NUCLEAR REACTIONS INTERACTION OF RADIATION AND MATTER During the radioactive decay process, a particle and/or a photon are emitted from the parent atom. The particles emitted carry energy proportional with their mass and speed. The photons carry energy proportional to their frequency. The photons and the particles interact with the surrounding matter. This interaction depends on their type (alpha, beta, gamma, neutrons, etc.), mass, electrical charge, energy, and on the composition of the surrounding materials. X-ray photons are created by the interaction of energetic electrons with matter at the atomic level. Photons (x-ray and gamma) end their lives by transferring their energy to electrons contained in matter. X-ray interactions are important in diagnostic examinations for many reasons. For example, the selective interaction of x-ray photons with the structure of the human body produces the image; the interaction of photons with the receptor converts an x-ray or gamma image into one that can be viewed or recorded. This chapter considers the basic interactions between x-ray and gamma photons and matter. TYPES OF INTERACTIONS ⮚ PHOTON INTERACTION Photons are individual units of energy. As an x-ray beam or gamma radiation passes through an object, three possible fates await each photon, as shown in the figure at the right side: 1. It can penetrate the section of matter without interacting. 2. It can interact with the matter and be completely absorbed by depositing its energy. 3. It can interact and be scattered or deflected from its original direction and deposit part of its energy. Photons Entering the Human Body Will Either Penetrate, Be Absorbed, or Produce Scattered Radiation There are two kinds of interactions through which photons deposit their energy; both are with electrons. In one type of interaction the photon loses all its energy; in the other, it loses a portion of its energy, and the remaining energy is scattered. These two interactions are shown below. Photoelectric ▪ A photon transfers all its energy to an electron which is located on one of the atomic shells. ▪ The following are the effects: o Electrons are ejected from the atom and starts to pass to a nearby matter. o Causing it to rapidly lose its energy and transfer in a short distance from its original location. o The energy of the photon then will be deposited in the matter which is close to the location of the photoelectric interaction. ▪ Basis for the reaction to occur: o When electrons are firmly bound to the atom o With a high binding energy which is a probable reason, they should be only slightly less than the energy of the photon, otherwise it will not occur. o Take note that this interaction is possible only when the photon has sufficient energy to overcome the binding energy and remove the electron from the atom. Compton Interaction ▪ In this interaction a portion of energy is absorbed only and the photon is formed with reduced energy. ▪ This photon leaves the site of the interaction in a direction different from that of the original photon. ▪ Because of the change in photon direction, this type of interaction is classified as a scattering process. In effect, a portion of the incident radiation "bounces off' or is scattered by the material. Coherent Scatter ▪ This is pure scattering interaction and there is no energy deposits in the material. This is possible only when the photon produces low energy and it cannot be used in most diagnostic procedure. Pair Production ▪ It only occurs in photons with energies in excess of 1.02 MeV. ▪ In this interaction the photon interacts with the nucleus wherein its energy is converted to matter. It produces a pair of particles which are the electron and a positively charged positron, they have the same mass, and each has an equivalent to a mass energy of 0.51 MeV. ▪ Like coherent scatter this is not used in diagnostic procedure as well. POSITRON INTERACTION Positron has a positive charge and is the same in size with that of the electron and it is different from an electron because they are composed of antimatter. This leads to a type of interaction that is quite different from the interactions among electrons. The interaction between a positron and matter is in two phases: ▪ ionization o as the energetic positron passes through matter, it interacts with the atomic electrons by electrical attraction. As the positron moves along, it pulls electrons out of the atoms and produces ionization. A small amount of energy is lost by the positron in each interaction. In general, this phase of the interaction is not too unlike the interaction of an energetic electron, but the positron pulls electrons as it races by and electrons push electrons away-from the path. Also, when the positron has lost most of its kinetic energy and is coming to a stop, it comes into close contact with an electron and enters into an annihilation interaction. ▪ Annihilation. o The annihilation process occurs when the antimatter positron combines with the conventional-matter electron. In this interaction, the masses of both particles are completely converted into energy. o The relationship between the amount of energy and mass is given by E = mc2 o The energy equivalent of one electron or positron mass is 511 keV. The energy that results from the annihilation process is emitted from the interaction site in the form of two photons, each with an energy of 511 keV. o The pair of photons leave the site in opposite directions. With special imaging equipment it is possible to capture both photons and to determine the precise threedimensional location of the interaction site. Since the range of a positron, like that of an electron, is relatively short, the site of interaction is always very close to the location of the radioactive nuclei. FUELS Fuel is a substance which gives heat energy on combustion. A fuel contains carbon and hydrogen as main combustible elements. Fuel is any material that can be made to react with other substances so that it releases chemical or nuclear energy as heat or to be used for work. Heat energy released by reactions of fuels is converted into mechanical energy via a heat engine. Other times the heat itself is valued for warmth, cooking, or industrial processes, as well as the illumination that comes with combustion. Fuels are also used in the cells of organisms in a process known as cellular respiration, where organic molecules are oxidized to release usable energy. CLASSIFICATION OF FUEL o BASED ON PHYSICAL STATE o BASED ON OCCURENCE BASED ON PHYSICAL STATE Liquid Fuels ▪ Liquid fuels like furnace oil are predominantly used in industrial applications. Most liquid fuels in widespread use are derived from the fossilized remains of dead plants and animals by exposure to heat and pressure in the Earth's crust. However, there are several types, such as hydrogen fuel (for automotive uses), ethanol, jet fuel and biodiesel which are all categorized as a liquid fuel. ▪ Types of liquid fuel o Petroleum o Oils from distillation of petroleum o Coal tar o Shale-oil o Alcohols, etc. ▪ liquid fuels has the following advantages and Disadvantages: Advantages: possess higher calorific value per unit mass as compare to solid fuels. out any loss. cost of liquid fuel is relatively much higher as compared to solid fuel. Disadvantages: bad odour. ners and spraying apparatus are required for efficient burning of liquid fuels. Solid Fuels ▪ Solid fuel refers to various types of solid material that are used as fuel to produce energy and provide heating, usually released through combustion. ▪ Coal is classified into three major types; anthracite, bituminous, and lignite. However, there is no clear demarcation between them. Coal is further classified as semi-anthracite, semi-bituminous, and sub-bituminous. Anthracite is the oldest coal from a geological perspective. It is a hard coal composed mainly of carbon with little volatile content and practically no moisture. ▪ Types of solid fuel o Wood, Coal Oilshal Tanbark, Bagasse,Straw, Charcoal, Coke, Briquettes ▪ Solid fuels has the following advantages and Disadvantages: Advantages explosion. burn with clinker formation. Disadvantages Gaseous Fuel ▪ Fuel gas is any one of a number of fuels that under ordinary conditions are gaseous. Many fuel gases are composed of hydrocarbons, hydrogen, carbon monoxide, or mixtures thereof. Such gases are sources of potential heat energy or light energy that can be readily transmitted and distributed through pipes from the point of origin directly to the place of consumption. Fuel gas is contrasted with liquid fuels and from solid fuels, though some fuel gases are liquefied for storage or transport. While their gaseous nature can be advantageous, avoiding the difficulty of transporting solid fuel and the dangers of spillage inherent in liquid fuels, it can also be dangerous. ▪ Types of gaseous fuel o Natural gas o Liquefied Petroleum gas (LPG) o Refinery gases o Methane from coal mines o Fuel gases made from solid fuel o Gases derived from coal o Gases derived from waste and biomass o Blast furnace gas o Gases made from petroleum o Gases from oil gasification o Gases from some fermentation process ▪ Gaseous fuels has the following advantages and Disadvantages over solid or liquid fuels : Advantages transportation. have high heat contents therefore provides higher temperatures. -heated by the heat of hot waste gases. Disadvantages PROPERTIES OF GASEOUS FUELS The fuel should be compared based on their net calorific value and especially true for natural gas because increased hydrogen content results in high water formation during combustion. 1. LPG ▪ LPG may be defined as those hydrocarbons, which are gaseous at normal atmospheric pressure but may be condensed to the liquid state at normal temperature by the application of moderate pressures. The LPG is a predominant mixture of propane and butane with a small percentage of unsaturated, some lighter C2 and heavier C5 fractions. The propane (C3H8), Propylene (C3H6), iso-butane (C4H10) and Butylene (C4H8) are included in the range of LPG. The liquid LPG evaporates to produce about 250 times volume of gas. ▪ LPG vapor is denser than air for example butane is about two times heavier then air and propane is about 1.5 times heavier then air. Consequently the vapors may flow along the ground and into drains sinking to the lowest level of the surroundings and be ignited at a considerable distance from the source of leakage. There should be adequate ground level of ventilation where LPG is stored therefore LPG cylinders should not be stored in cellars or basements which have no ventilation at ground level. 2. Natural gas ▪ Natural gas has high calorific value and requiring no storage facilities. It mixes with air readily and does not produce smoke or soot. It did not contains Sulphur. It is lighter than air and disperses into air easily in case of leak. ▪ Methane is the main constituent of natural gas and it is about 95% of the total volume. The other components are Ethane, Propane, Butane, Pentane, Nitrogen, Carbon Dioxide, and traces of other gases. In these gases a very small amounts of sulphur compounds are also present. The properties of methane are used when comparing the properties of natural gas to other fuels because methane is the largest component in natural gas. BASED ON OCCURENCE ▪ PRIMARY OR NATURAL FUEL wood coal ▪ SECONDARY OR PREPARED FUELS charcoal petroleum