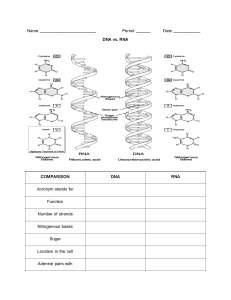
Chapter 1. Principles of Biochemistry 1.1 What Is Biochemistry? 1.2 The Chemical Basis of Life: A Hierarchical Perspective 1.3 Storage and Processing of Genetic Information 1.4 Determinants of Biomolecular Structure and Function 1.1 What Is Biochemistry? § Biochemistry aims to explain biological processes at the molecular and cellular levels. § It is a core discipline in life sciences. § It is at the interface of biology and chemistry. § It relies heavily on the quantitative analysis of data. § It often studies in vitro (outside a living cell) systems. It All Started with Fermentation… § Fermentation is the conversion of rotting fruit or grain into alcohol solutions through the action of yeast. § Yeast was determined to be the catalyst. § Used to produce wine and beer from yeast Alcoholic Fermentation § This reaction has been around since 2000 B.C. § Buchner demonstrated that CO2 and CH3CH2OH were produced in vitro from sugar using brewer’s yeast in 1897. § Buchner is credited with proposing that “enzymes” helped speed up this reaction. Catalysts § Biomolecules that increase the rate (catalyze) of biochemical reactions dramatically § Found in all living cells § Responsible for the following reactions: • Aerobic respiration • Fermentation • Nitrogen metabolism • Energy conversion • Programmed cell death § Examples: • Proteins or ribonucleic acid (RNA) Biochemistry: An Applied Science § Biochemistry uses advanced experimental methods to develop in vitro conditions for exploiting cellular processes and enzymatic reactions. 1.2 The Chemical Basis of Life: A Hierarchical Perspective § The foundation of this hierarchy includes chemical elements and functional groups. § Chemical elements: Element Symbol Percent dry weight (percent) 62 Additional trace elements (less than 0.1 percent), element Manganese Additional trace elements (less than 0.1 percent), Symbol Upper M, n Carbon Upper C Nitrogen Upper N 11 Iron Upper F, e Oxygen Upper O 9 Cobalt Upper C, o Hydrogen Upper H 6 Copper Upper C, u Calcium Upper C, a 5 Zinc Upper Z, n Phosphorous Upper P 3 Selenium Upper S, e Potassium Upper K 1 Molybdenum Upper M, o Sulfur Upper S 1 Iodine I Chlorine Upper C, l Less than 1 Fluorine Upper F Sodium Upper N a Less than 1 Chromium Upper C, r Magnesium Upper M, g Less than 1 Tin Upper S, n Organizational Hierarchy of Biochemistry Chemical Bonding Observed in Biochemistry § The most common carbon bonds are C—C, C=C, C—H, C=O, C—N, C—S, and C—O. Atom Number of unpaired electrons Upper H, one unpaired atom 1 Upper O, four paired and two unpaired atom. 2 Upper N, two paired and three unpaired atom. 3 Upper C four unpaired atom. 4 Molecular Geometry Revisited § A carbon atom can bind up to four single bonds to form a tetrahedron. § The rotation around a single bond is very easy due to its sigma bond, whereas a carbon–carbon double bond includes a pi bond and rotation is not possible without breaking this pi bond. Trace Elements § In addition to the elements observed in Table 1.1, trace elements are used as cofactors in proteins and are required for life. § These elements are required in smaller (“trace”) amounts. § These elements include: • Zinc • Iron • Manganese • Copper • Cobalt Essential Ions § Play a key role in cell signaling and neurophysiology § Include: • Calcium • Chloride • Magnesium • Potassium • Sodium Functional Groups § Play an important role in structure and function of biomolecules Biomolecules, Part 1 § Four major types: • Amino acids • Nucleotides • Simple sugars • Fatty acids Biomolecules, Part 2 § Primary cellular function § Amino acid § § § • Protein function • Neurotransmission • Nitrogen metabolism • Energy conversion Nucleotides • Nucleic acid function • Energy conversion • Signal transduction • Enzyme catalysis Simple sugar • Energy conversion • Cell wall structure • Cell recognition • Nucleotide structure Fatty acid • Cell membranes • Energy conversion • Cell signaling Amino Acids § Nitrogen-containing molecules that function primarily as the building blocks of protein § Covalently linked into a linear chain to form polypeptides § Differ from each other by the side chain attached at the central carbon Nucleotides § Include the nucleic acids, DNA and RNA § Consist of the following: • Nitrogenous base • Five-membered sugar • 1–3 phosphate groups § Examples include: • Cytosine • ATP • cAMP • NAD+ Simple Sugars § Carbohydrates • Contain C, H, and O atoms only § Have a 2:1 ratio of hydrogen atoms to oxygen atoms § Include: • Monosaccharides • Disaccharides Fatty Acids § Amphipathic molecules § Act as components of plasma membrane lipids § Act as a storage form of energy (i.e., fats) § Consist of: • Carboxyl group attached to a hydrocarbon chain Saturated vs. Polyunsaturated Fatty Acids § Saturated fatty acids contain no C=C double bonds in the hydrocarbon chain. § Polyunsaturated fatty acids contain multiple C=C double bonds in the hydrocarbon chain. Macromolecules § Higher-end structural form of biomolecules § Include: • Chemical polymers such as: – Proteins—amino acid polymers – Nucleic acids—nucleotide polymers – Polysaccharides—polymers of glucose molecules Polymers in Macromolecules: Nucleic Acids § Covalently linked nucleotides § Include DNA and RNA § Nucleotides are linked together by phosphodiester bonds. Polymers in Macromolecules: Proteins § Covalently linked amino acids § Also known as polypeptides • R = different amino acid side chains Polymers in Macromolecules: Polysaccharides § Consist of mixtures of simple sugars of repeating units of glucose § Covalent linkage between glucose units (i.e., glycosidic bond) is key to the identification and chemical properties of the polysaccharide. Various Examples of Polysaccharides Metabolic Pathways § Enable cells to coordinate and control complex biochemical processes in response to available energy § Function within membrane-bound cells § Examples include: • Glycolysis and gluconeogenesis (glucose metabolism) • Citrate cycle (energy conversion) • Fatty acid oxidation and biosynthesis (fatty acid metabolism) Metabolic Pathway Terminology § Metabolites • Small biomolecules that serve as both reactants and products in biochemical reactions within cells • Frequently observed in reactions that are essential in lifesustaining processes § Metabolic flux • The rate at which reactants and products are interconverted in a metabolic pathway Metabolic Pathway Example: The Urea Cycle Metabolic Pathway Formats Cellular Structures Key Cellular Structure Functions, Part 1 § Genome • All encoded genes and other DNA elements specifying genetic composition of prokaryotic and eukaryotic cells § Nucleolus • Site of ribosome assembly § Ribosomes • Location of protein synthesis Key Cellular Structure Functions, Part 2 § Mitochondria • Responsible for ATP production § Peroxisomes and lysosomes • Involved in macromolecule degradation and detoxification § Endoplasmic reticulum • Sequester ribosomes for protein synthesis § Golgi apparatus • Involved in protein translocation and protein secretion in the plasma membrane Cell Specialization § A higher level of organizational complexity § Allows multicellular organisms to exploit their environment through signal transduction Signal Transduction Organisms § A complex organization level that consists of specialized cells § Allow multicellular organisms to respond to environmental changes § Can adapt to change through signal transduction mechanisms that facilitate cell–cell communication The Circulatory System Ecosystems § Highest level of hierarchical organization § Include cohabitation of different organisms in the same environmental niche § Involve a shared use of resources and waste management Ecosystem Examples § This is the top rung of the hierarchal ladder of life. § This is how organisms interact with their environment and each other. 1.3 Storage and Processing of Genetic Information § 1952 – DNA was determined to be sufficient to promote viral replication. § Rosalind Franklin collected Xray diffraction data to determine the structure of DNA. Watson and Crick’s Discovery § 1953 – Watson and Crick determined that DNA is a double helix. § This discovery explained how DNA was used to pass on genetic material. § 1962 – The duo were awarded the Nobel Prize in Physiology or Medicine. Deoxyribonucleotides vs. Ribonucleotides § Deoxyribonucleotides are monomeric units of DNA that lack an OH group on the C-2' of the ribose sugar. § Ribonucleotides are structurally similar to deoxyribonucleotides, except they contain an OH at the C-2' position in the ribose sugar. Nucleotide Base Pairs § The complimentary base pairs are as follows: • in DNA: G-C and A-T • in RNA: G-C and A-U Nucleotide Base Pairs in the Helix Central Dogma § Describes how information is transferred between DNA, RNA, and protein Relationship between DNA and Protein “-ome” Biochemistry § Genome • Collection of genes § Transcriptome • Collection of DNA transcripts (RNA products) generated by DNA transcription § Proteome • Collection of proteins produced by mRNA translation either in the entire organism or under special conditions 1.4 Determinants of Biomolecular Structure and Function § Structure determines function for DNA. Mutant Genes § Proteins acquire a bounty of molecular structures through random mutations. Mutations § Can be germ-line cell • Passed from parents to offspring • Result in inherited genetic diseases § Can be in somatic cells • Not inherited by the offspring • Limited to the individual organism Random Mutation and Natural Selection Gene Duplications