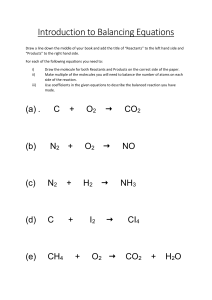
Lesson 1 Reactions and Equations Focus Question How are chemical reactions modeled? New Vocabulary chemical reaction reactant product chemical equation coefficient Review Vocabulary chemical change: a process involving one or more substances changing into a new substance Chemical Reactions • The process by which the atoms of one or more substances are rearranged to form different substances is called a chemical reaction. • A chemical reaction is another name for a chemical change. Chemical Reactions Evidence of a Chemical Reaction • A temperature change can indicate a chemical reaction. Many reactions release energy in the form of heat and light. Other chemical reactions absorb heat. • Color change can indicate a chemical reaction. • Odor, gas bubbles, and the formation of a solid are other indications of chemical change. Representing Chemical Reactions • Equations are used to represent chemical reactions. • Equations show a reaction’s reactants (starting substances) and products (substances formed). • The table shows the symbols used in chemical equations. Representing Chemical Reactions Word Equations aluminum(s) + bromine(l) → aluminum bromide(s) • In this word equation, aluminum(s) + bromine(l) → aluminum bromide(s) reads as “aluminum and bromine react to produce aluminum bromide.” • Word equations lack information about the number of atoms involved. Representing Chemical Reactions Skeleton Equations Al(s) + Br(l) → AlBr3(s) • Skeleton equations use symbols and formulas to represent reactants and products. • These equations also lack information about the number of atoms involved. Representing Chemical Reactions Chemical Equations A chemical equation uses chemical formulas to show the identities and relative amounts of the substances involved in a chemical reaction. Balancing Chemical Equations A coefficient in a chemical equation is the number written in front of a reactant or product. Balancing Chemical Equations Steps for Balancing Equations 1. Write the skeleton equation for the reaction. 2. Count the atoms of the elements in the reactants. 3. Count the atoms of the elements in the products. 4. Change the coefficients to make the number of each element equal on both sides of the equation. This shows that atoms are conserved. 5. Write the coefficients in their lowest possible ratio. 6. Check your work. Writing a Balanced Chemical Equation Use with Example Problem 1. Problem Write the balanced chemical equation for the reaction in which aqueous sodium hydroxide and aqueous calcium bromide react to produce solid calcium hydroxide and aqueous sodium bromide. SOLVE FOR THE UNKNOWN Write the skeleton equation for the chemical reaction. Be sure to put the reactants on the left side of the arrow and the products on the right. Separate the substances with plus signs, and indicate their physical states. NaOH(aq) + CaBr2(aq) → Ca(OH)2(s) + NaBr(aq) • Response ANALYZE THE PROBLEM You are given the reactants and products in a chemical reaction. Start with a skeleton equation, and use the steps for balancing chemical equations. Count the atoms of each element in the reactants. 1 Na, 1 O, 1 H, 1 Ca, 2 Br • Count the atoms of each element in the products. 1 Na, 2 O, 2 H, 1 Ca, 1 Br Writing a Balanced Chemical Equation SOLVE FOR THE UNKNOWN • Insert the coefficient 2 in front of NaOH to balance the hydroxide ions. 2NaOH + CaBr2 → Ca(OH)2 + NaBr • Insert the coefficient 2 in front of NaBr to balance the Na and Br atoms. 2NaOH + CaBr2 → Ca(OH)2 + 2NaBr • Write the coefficients in their lowestpossible ratio. The ratio of the coefficients is 2:1:1:2. • Check to make sure that the number of atoms of each element is equal on both sides of the equation. Reactants: 2 Na, 2 OH, 1 Ca, 2 Br Products: 2 Na, 2 OH, 1 Ca, 2 Br. EVALUATE THE ANSWER The chemical formulas for all substances are written correctly. The number of atoms of each element is equal on both sides of the equation. The coefficients are written in the lowest possible ratio. The balanced chemical equation for the reaction is 2NaOH(aq) + CaBr2(aq) → Ca(OH)2(s) + 2NaBr(aq) Quiz 1. Which of the following is not an example of a chemical reaction? A rusting iron B burning wood C boiling water D rotting food CORRECT Quiz 2. Which of the following is not true about coefficients in a balanced chemical equation? A They are numbers written in front of reactants or products. C They describe the highest whole number ratio of the amounts of all reactants and products. CORRECT B They are usually whole numbers. D They are not usually written if the value is 1. Quiz 3. Which observation is NOT physical evidence that a chemical reaction has occurred? A There is an odor change. B A solid is no longer magnetic. C A solid melts. D A solid changes color. CORRECT Quiz 4. Which of the following is NOT true about balancing chemical equations? A Subscripts should never be changed to balance the equation. C Coefficients are changed to make the number of each element equal on both sides. B Coefficients are written in their lowest possible ratio. D Subscripts are changed to make the number of each element equal on both sides. CORRECT