Gene Mutation, DNA Repair & Recombination - Genetics
advertisement

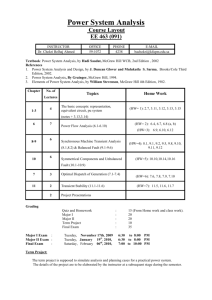
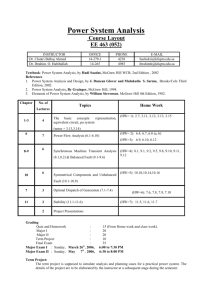
Because learning changes everything. ® Chapter 19 Gene Mutation and DNA Repair, and Recombination Genetics: Analysis & Principles SEVENTH EDITION Robert J. Brooker © 2021 McGraw Hill. All rights reserved. Authorized only for instructor use in the classroom. No reproduction or further distribution permitted without the prior written consent of McGraw Hill. Introduction 1 The term mutation refers to a heritable change in the genetic material Mutations provide allelic variation • On the positive side, mutations are the foundation for evolutionary change needed for a species to adapt to changes in the environment • On the negative side, new mutations are much more likely to be harmful than beneficial to the individual and often are the cause of diseases © McGraw Hill 2 Introduction 2 Understanding the molecular nature of mutations is a deeply compelling area of research Since mutations can be quite harmful, organisms have developed ways to repair damaged DNA Another topic explored in this chapter is homologous recombination, which involves exchange of identical or similar DNA segments between homologous chromosomes; enhances genetic diversity; involved in DNA repair © McGraw Hill 3 19.1 Effects of Mutations on Gene Structure and Function Mutations can be: • Changes in chromosome structure • Changes in chromosome number • Changes in DNA of a single gene • Discussed in this chapter • Mutations can affect the molecular and phenotypic expression of genes © McGraw Hill 4 Gene Mutations 1 Gene mutations are molecular changes in the DNA sequence of a gene A point mutation is a change in a single base pair • It can involve a base substitution • 5’ AACGCTAGATC 3’ → 5’ AACGCGAGATC 3’ • A transition is a change of a pyrimidine (C, T) to another pyrimidine or a purine (A, G) to another purine • A transversion is a change of a pyrimidine to a purine or vice versa • Transitions are more common than transversions © McGraw Hill 5 Gene Mutations 2 Mutations may also involve the addition or deletion of short sequences of DNA 5’ AACGCTAGATC 3’ → 3’ TTGCGATCTAG 5’ 5’ AACGCTC 3’ 3’ TTGCGAG 5’ 5’ AACGCTAGATC 3’ → 3’ TTGCGATCTAG 5’ 5’ AACAGTCGCTAGATC 3’ 3’ TTGTCAGCGATCTAG 5’ © McGraw Hill 6 Gene Mutations Can Alter the Coding Sequence Within a Gene 1 Mutations in the coding sequence of a protein-encoding gene can have various effects on the polypeptide • Silent mutations (mutaciones silenciosas) are those base substitutions that do not alter the amino acid sequence of the polypeptide • Due to the degeneracy of the genetic code • Missense mutations (mutación con cambio de sentido) are those base substitutions in which an amino acid change does occur • Example: Sickle cell disease (Refer to Figure 19.1) • Unlike sickle cell disease, a missense mutation may have no detectable effect on protein function, and the mutation is said to be neutral. This is more likely to occur if the new amino acid has similar chemistry to the amino acid it replaced © McGraw Hill 7 Figure 19.1 © McGraw Hill (a-b): ©Science History Images/Alamy 8 Missense Mutation in Sickle Cell Disease (anemia falciformes) A comparison of the amino acid sequence between normal β globin and sickle cell β globin: Normal: NH2 – VALINE – HISTIDINE – LEUCINE – THREONINE – PROLINE – GLUTAMIC ACID – GLUTAMIC ACID… Sickle cell: NH2 – VALINE – HISTIDINE – LEUCINE – THREONINE – PROLINE – VALINE – GLUTAMIC ACID… © McGraw Hill 9 Gene Mutations Can Alter the Coding Sequence Within a Gene 2 Mutations in the coding sequence of a protein-encoding gene can have various effects on the polypeptide • Nonsense mutations (mutación sin sentido) are those base substitutions that change a normal codon to a stop codon • Frameshift mutations (cambio del marco de lectura) involve the addition or deletion of a number of nucleotides that is not divisible by three • This shifts the reading frame so that translation of the mRNA results in a completely different amino acid sequence downstream of the mutation © McGraw Hill 10 Gene Mutations Can Alter the Coding Sequence Within a Gene 3 Access the text alternative for slide images. © McGraw Hill 11 Gene Mutations Outside of Coding Sequences 1 Gene mutations outside of coding sequences can affect gene expression and phenotype Mutations in the core promoter can change levels of gene transcription • Up promoter mutations increase transcription • Down promoter mutations decrease transcription © McGraw Hill 12 Gene Mutations Outside of Coding Sequences 2 TABLE 19.2 Possible Consequences of Gene Mutations Outside of a Coding Sequence Sequence Effect of Mutation Promoter May increase or decrease the rate of transcription Regulatory element/operator site May disrupt the ability of the gene to be properly regulated 5'-UTR/3'-UTR May alter the ability of mRNA to be translated; may alter mRNA stability Splice recognition sequence May alter the ability of pre-mRNA to be properly spliced © McGraw Hill 13 Gene Mutations Are Given Names That Describe How They Affect Genotype and Phenotype In a natural population, the wild-type is the relatively prevalent genotype. Genes with multiple alleles may have two or more wild-types. • A forward mutation changes the wild-type genotype into some new variation • A reverse mutation changes a mutant allele back to the wild-type • It is also termed a reversion © McGraw Hill 14 Mutations Based on Their Effects on Wild-Type Phenotype 1 Mutations can also be described based on their effects on the wild-type phenotype They are often characterized by their differential ability to survive • Deleterious mutations decrease the chances of survival • The most extreme are lethal mutations • Beneficial mutations enhance the survival or reproductive success of an organism • The environment can affect whether a given mutation is deleterious or beneficial © McGraw Hill 15 Mutations Based on Their Effects on Wild-Type Phenotype 2 Some mutations are conditional • They affect the phenotype only under a defined set of conditions • An example is a temperature-sensitive mutation © McGraw Hill 16 Suppressor Mutations Suppressor mutations reverse the phenotypic effects of another mutation A second mutation will sometimes counteract the effects of a first mutation These second-site mutations are called suppressor mutations or simply suppressors different to reversion Intragenic suppressors • The second mutation is within the same gene as the first mutation • Typically, the first mutation causes an abnormality in protein structure and second mutation restores normal protein structure © McGraw Hill 17 Intragenic Suppressor Mutations 1 TABLE 19.3 Examples of Suppressor Mutations Type: Intragenic No Mutation Transport can occur © McGraw Hill 18 Intragenic Suppressor Mutations 2 First Mutation (x) Transport inhibited Second Mutation (x) Transport can occur Description A first mutation disrupts normal protein function, and a suppressor mutation affecting the same protein restores function. In this example, the first mutation inhibits transport function, and the second mutation restores it. © McGraw Hill 19 Intergenic Suppressors • The second mutation is in a different gene from the first mutation • Examples: • Redundant function • Common pathway • Multimeric proteins • Transcription factors © McGraw Hill 20 Intergenic Suppressor – Redundant Function Redundant function A first mutation inhibits the function of a protein, and a second mutation alters a different protein to carry out that function. In this example, the proteins function as enzymes. Access the text alternative for slide images. © McGraw Hill 21 Intergenic Suppressor – Common Pathway Common pathway Two or more different proteins may function as enzymes in a common pathway. A mutation that causes a defect in one enzyme may be compensated for by a mutation that increases the function of a different enzyme in the same pathway. Access the text alternative for slide images. © McGraw Hill 22 Intergenic Suppressor – Multimeric Protein Multimeric protein A mutation in a gene encoding one protein subunit that inhibits function may be suppressed by a mutation in a gene that encodes a different subunit. The double mutant has restored function. Access the text alternative for slide images. © McGraw Hill 23 Intergenic Suppressor – Transcription Factor Transcription factor A first mutation causes loss of function of a particular protein. A second mutation may alter a transcription factor and cause it to activate the expression of another gene. This other gene encodes a protein that can compensate for the loss of function caused by the first mutation. Access the text alternative for slide images. © McGraw Hill 24 Changes in Chromosome Structure Can Affect Gene Expression A chromosomal rearrangement may affect a gene because the chromosomal breakpoint (site of breaking and rejoining) occurs within the gene inversion or translocation Alternatively, a gene may be left intact, but its expression may be altered because of its new location • This is termed a position effect There are two common reasons for position effects: 1. Movement to a position next to regulatory sequences 2. Movement to a heterochromatic region © McGraw Hill 25 Figure 19.2a (a) Position effect due to regulatory sequences Regulatory sequences are often bidirectional, so gene A may now show the expression pattern of gene B Access the text alternative for slide images. © McGraw Hill 26 Figure 19.2b (b) Position effects due to translocation to a heterochromatic chromosome Access the text alternative for slide images. © McGraw Hill 27 Figure 19.3 (a) Normal eye © McGraw Hill (b) Variegated eye (a-b): © Dr. Jack R. Girton 28 Mutations can Occur in Germ-Line or Somatic Cells Geneticists classify animal cells into two types • Germ-line cells • Cells that give rise to gametes such as eggs and sperm • Somatic cells • All other cells Germ-line mutations are those that occur directly in a sperm or egg cell, or in one of their precursor cells Somatic mutations are those that occur directly in a body cell that is not part of the germ-line © McGraw Hill 29 Effects of Germ-Line and Somatic Mutations Germ-line mutations occur in gametes • Passed to half of the gametes in the next generation; mutation found in whole body Somatic mutations result in patches of affected area • The size of the patch will depend on the timing of the mutation. The earlier the mutation, the larger the patch • An individual with somatic regions that are genotypically different from the rest of the body is called a genetic mosaic • Mutations not present in gametes © McGraw Hill 30 Figure 19.4 Access the text alternative for slide images. © McGraw Hill 31 19.2 Random Nature of Mutations Are mutations spontaneous occurrences or causally related to environmental conditions? This is a question that biologists have asked themselves for a long time Jean Baptiste Lamarck-physiological adaptation • Proposed that physiological events (for example use and disuse) determine whether traits are passed along to offspring Alternative possibility-random mutations • Genetic variation occurs by chance • Natural selection results in organisms with greater reproductive success © McGraw Hill 32 Experiment 19A: Testing the Random Mutation Theory 1 Joshua and Esther Lederberg studied the resistance of E. coli to infection by bacteriophage T1 • tonr (T one resistance) • They asked if tonr is due to spontaneous mutations that occur at a low rate or to a physiological adaptation • The physiological adaptation hypothesis predicts that the number of tonr bacteria is very low unless there is selection for T1 resistance © McGraw Hill 33 Experiment 19A: Testing the Random Mutation Theory 2 • The random mutation hypothesis predicts that mutations will happen randomly and will occur without selection Hypothesis: Mutations are random events © McGraw Hill 34 Experiment 19A Steps 1. Place individual bacterial cells onto growth media. 2. Incubate overnight to allow the formation of bacterial colonies. This is called the master plate. 3. Press a velvet cloth (wrapped over a cylinder) onto the master plate, and then lift gently to obtain a replica of each bacterial colony. Press the replica onto 2 secondary plates that contain T1 phage. Incubate overnight to allow growth of mutant cells. © McGraw Hill 35 Figure 19.6 Access the text alternative for slide images. © McGraw Hill 36 Interpreting the Data Resistant cells were in the same location on both plates. • Mutations had occurred randomly in the absence of selection by T1 (master plate) • Became observable with selection but new colonies did not appear due to the presence of T1 • Supports random mutation hypothesis, now called the random mutation theory © McGraw Hill 37 19.3 Spontaneous Mutations Mutations can occur spontaneously or be induced Spontaneous mutations • Result from abnormalities in cellular/biological processes • Errors in DNA replication, for example • Underlying cause originates within the cell Induced mutations • Caused by environmental agents • Agents that are known to alter DNA structure are termed mutagens • These can be chemical (smoke) or physical agents (UV light) Refer to Table 19.4 in your textbook © McGraw Hill 38 Causes of Spontaneous Mutations Spontaneous mutations can arise by three types of chemical changes: 1. Depurination 2. Deamination 3. Tautomeric shift © McGraw Hill 39 Depurination The removal of a purine (guanine or adenine) from the DNA forms an apurinic site The covalent bond between deoxyribose and a purine base is somewhat unstable • It occasionally undergoes a spontaneous reaction with water that releases the base from the sugar • Mammalian cells lose approximately 10,000 purines per 24 hours at 37° Fortunately, apurinic sites can be repaired • However, if the repair system fails, a mutation may result during subsequent rounds of DNA replication • © McGraw Hill Polymerase will add a random base 40 Figure 19.7 (a) Depurination (b) Replication over an apurinic site During replication, three out of four bases (A, T and G) are the incorrect nucleotide 75% chance of a mutation Access the text alternative for slide images. © McGraw Hill 41 Deamination of Cytosine Removal of an amino group from the cytosine base • The other bases are not readily deaminated DNA repair enzymes can recognize uracil as an inappropriate base in DNA and remove it • However, if the repair system fails, a C-G to A-T mutation will result during subsequent rounds of DNA replication © McGraw Hill 42 Figure 19.8a (a) Deamination of cytosine © McGraw Hill 43 Deamination of 5-methylcytosine 5-methylcytosine can be deaminated into thymine, a normal constituent of DNA Repair enzymes cannot determine which of the two bases on the two DNA strands is the incorrect base For this reason, methylated cytosine bases tend to create hot spots for mutation Fig. 19.8b (b) Deamination of 5-methylcytosine © McGraw Hill 44 Tautomeric Shift (tautómeros) tauto = igual y meros = parte) A temporary change in base structure The common, stable form of thymine and guanine is the keto form • Rarely, T and G convert to an enol form The common, stable form of adenine and cytosine is the amino form • Rarely, A and C can convert to an imino form © McGraw Hill 45 Figure 19.9a (1) (a) Tautomeric shifts that occur in the 4 bases found in DNA © McGraw Hill 46 Figure 19.9a (2) © McGraw Hill 47 Figure 19.9b (b) Mistakes in base pairing © McGraw Hill 48 Figure 19.9c To cause mutation, a tautomeric shift must occur immediately prior to replication (c) Tautomeric shifts and DNA replication can cause mutation Access the text alternative for slide images. © McGraw Hill 49 Oxidative Stress May Also Lead to DNA Damage and Mutation 1 Aerobic organisms produce Reactive Oxygen Species (ROS) including• Hydrogen peroxide • Superoxide • Hydroxyl radical • Body tries to block buildup of ROS • Enzymes such as superoxide dismutase and catalase • Antioxidants Oxidative stress: an imbalance between the production of ROS and an organism’s ability to break them down © McGraw Hill 50 Oxidative Stress May Also Lead to DNA Damage and Mutation 2 ROS overaccumulation can lead to Oxidative DNA damage For example, guanine can be converted to 7,8-dihydro-8oxoguanine (abbreviated 8-oxoG) • Pairs with adenine during replication (transversion mutation) • Causes GC base pair → TA base pair Figure 19.10 © McGraw Hill 51 Mutations Due to Trinucleotide Repeats 1 Several human genetic diseases are caused by an unusual form of mutation called trinucleotide repeat expansion (TNRE) Certain regions of the chromosome contain trinucleotide sequences repeated in tandem • In individuals without disease symptoms, these sequences are transmitted from parent to offspring without mutation • However, in persons with TNRE disorders, the length of a trinucleotide repeat has increased above a certain critical size • Disease symptoms occur © McGraw Hill 52 Mutations Due to Trinucleotide Repeats 2 In some cases, the expansion is within the coding sequence of the gene Typically the trinucleotide expansion is CAG (glutamine) Therefore, the encoded protein will contain long tracks of glutamine • This causes the proteins to aggregate with each other • This aggregation is correlated with the progression of the disease, but may not cause disease symptoms © McGraw Hill 53 Mutations Due to Trinucleotide Repeats 3 In other cases, the expansions are located in noncoding regions of genes • Some of these expansions are hypothesized to cause abnormal changes in RNA structure • Some produce methylated CpG islands which may silence the gene © McGraw Hill 54 Mutations Due to Trinucleotide Repeats 4 Some TNRE disorders progressively worsen in future generations • May depend on which parent the mutant allele comes from • In Huntington disease, the TNRE is more likely to occur if inherited from the father • In myotonic muscular dystrophy, the TNRE is more likely to occur if inherited from the mother • This suggests that TNRE can occur more frequently during oogenesis or spermatogenesis, depending on the gene involved This is called anticipation © McGraw Hill 55 Mechanism of Trinucleotide Repeat Expansion 1 TNREs contain at least one C and one G • This allows formation of a hairpin During DNA replication, a hairpin can lead to an increase or decrease in the length of the DNA • Polymerase can slip off DNA • Hairpin forms and pulls strand back • DNA polymerase hops back on • Begins synthesis from new location © McGraw Hill 56 Mechanism of Trinucleotide Repeat Expansion 2 These changes can occur during gamete formation • Offspring will have very different numbers of repeats Can also increase repeats in somatic cells • This can increase severity of the disease with age © McGraw Hill 57 Figure 19.11a (a) Formation of a hairpin with a trinucleotide (CTG) repeat sequence © McGraw Hill 58 Figure 19.11b (b) Mechanism of trinucleotide repeat expansion Access the text alternative for slide images. © McGraw Hill 59 19.4 Induced Mutations Agents that alter the structure of DNA and thereby cause mutations are called mutagens The public is concerned about mutagens for two main reasons: 1. Mutagens are often involved in the development of human cancers 2. Mutagens can cause gene mutations that may have harmful effects in future generations An enormous array of agents can act as mutagens Mutagenic agents are usually classified as chemical or physical mutagens © McGraw Hill 60 Mutagens Alter DNA Structure in Different Ways Chemical mutagens come in three main types: 1. Base modifiers • Some covalently modify base structure • Others disrupt pairing by alkylating bases 2. Intercalating agents • Directly interfere with replication process 3. Base analogues • Incorporate into DNA and disrupt structure • Some tautomerize at a high rate Physical mutagens include radiation: • X-rays, gamma rays, ionizing radiation, UV light © McGraw Hill 61 Base Modifiers Base modifiers covalently modify the structure of a nucleotide For example, nitrous acid replaces amino groups with keto groups (–NH2 to =O) This can change cytosine to uracil and adenine to hypoxanthine • These modified bases do not pair with the appropriate nucleotides in the daughter strand during DNA replication Some chemical mutagens disrupt the appropriate pairing between nucleotides by alkylating bases within the DNA • Examples: Nitrogen mustards and ethyl methanesulfonate (EMS) © McGraw Hill 62 Figure 19.12 © McGraw Hill 63 Intercalating Agents Intercalating agents contain flat planar structures that intercalate themselves into the double helix • This distorts the helical structure • When DNA containing these mutagens is replicated, the daughter strands may contain single-nucleotide additions and/or deletions resulting in frameshifts • Examples: • Acridine dyes • Proflavin © McGraw Hill 64 Base Analogues Base analogues become incorporated into daughter strands during DNA replication For example, 5-bromouracil is a thymine analogue • It can be incorporated into DNA instead of thymine • A tautomeric shift can result in pairing with guanine © McGraw Hill 65 Figure 19.13a (a) Base pairing of 5BU (a thymine analog) with adenine or guanine © McGraw Hill 66 Figure 19.13b (b) How 5BU causes a mutation in a base pair during DNA replication Access the text alternative for slide images. © McGraw Hill 67 Types of Physical Mutagens 1 Ionizing radiation Includes X-rays and gamma rays • Has short wavelength and high energy • Can penetrate deeply into biological molecules • Creates chemically reactive molecules termed free radicals • Can cause • Base deletions • Oxidized bases • Single nicks in DNA strands • Cross-linking • Chromosomal breaks © McGraw Hill 68 Types of Physical Mutagens 2 Nonionizing radiation • Includes UV light • Has less energy • Cannot penetrate deeply into biological molecules • Causes the formation of cross-linked thymine dimers • Thymine dimers may cause mutations when that DNA strand is replicated Fig. 19.14 © McGraw Hill 69 Mutation Rates and Frequencies 1 The term mutation rate is the likelihood that a gene will be altered by a new mutation • Commonly expressed as the number of new mutations in a given gene per cell generation • Range of 10-5 to 10-9 per generation • Humans add 100-200 new mutations/generation The mutation rate for a given gene is not constant • It can be increased by the presence of mutagens Mutation rates vary substantially between species and even within different strains of the same species © McGraw Hill 70 Mutation Rates and Frequencies 2 The mutation frequency for a gene is the number of mutant genes divided by the total number of genes in a population If 1 million bacteria were plated and 10 were mutant • The mutation frequency would be 1 in 100,000 or 10-5 The mutation frequency is important in areas of genetics such as population genetics • Mutation frequencies may become greater than mutation rates • Due to natural selection and genetic drift © McGraw Hill 71 Testing Methods can Determine if an Agent is a Mutagen Many different tests have been used to evaluate mutagenicity One commonly used test is the Ames test • Developed by Bruce Ames • The test uses a strain of Salmonella typhimurium that cannot synthesize the amino acid histidine • It has a point mutation in a gene involved in histidine biosynthesis • A second mutation (that is, a reversion) may occur, thereby restoring the ability to synthesize histidine • The Ames test monitors the rate at which this second mutation occurs © McGraw Hill 72 The Ames Test for Mutagenicity 1 Rat liver extract provides a mixture of enzymes that may activate a mutagen Different strains with transition, transversion or frameshift mutations can be used The control plate indicates that there is a low level of spontaneous mutation © McGraw Hill 73 The Ames Test for Mutagenicity 2 Mix together the suspected mutagen, a rat liver extract, and a Salmonella strain that cannot synthesize histidine. The suspected mutagen is omitted from the control sample. Figure 19.15 Access the text alternative for slide images. © McGraw Hill 74 19.5 DNA Repair Because most mutations are deleterious, DNA repair systems are vital to the survival of all organisms • Living cells contain several DNA repair systems that can fix different type of DNA alterations • See Table 19.7 in your textbook In most cases, DNA repair is a multi-step process 1. An irregularity in DNA structure is detected 2. The abnormal DNA is removed 3. Normal DNA is synthesized © McGraw Hill 75 Damaged Bases Can Be Directly Repaired In a few cases, the covalent modifications of nucleotides can be reversed by specific enzymes Photolyase can repair thymine dimers • It splits the dimers restoring the DNA to its original condition • Uses energy of visible light for photoreactivation Alkyltransferase repairs alkylated bases • It transfers the methyl or ethyl group from the base to a cysteine side chain within the alkyltransferase protein • Surprisingly, this permanently inactivates alkyltransferase © McGraw Hill 76 Figure 19.16a (a) Direct repair of a thymine dimer © McGraw Hill 77 Figure 19.16b (b) Direct repair of a methylated base © McGraw Hill 78 Base Excision Repair Removes a Damaged Base Base excision repair (BER) involves a category of enzymes known as DNA N-glycosylases • These enzymes can recognize an abnormal base and cleave the bond between it and the sugar in the DNA Depending on the species, this repair system can eliminate abnormal bases such as • Uracil; 3-methyladenine; 7-methylguanine © McGraw Hill 79 Figure 19.17 Access the text alternative for slide images. © McGraw Hill 80 Nucleotide Excision Repair Removes Damaged DNA Segments 1 An important general process for DNA repair is nucleotide excision repair (NER) This type of system can repair many types of DNA damage, including • Thymine dimers and chemically modified bases • Missing bases • Some types of crosslinks NER is found in all eukaryotes and prokaryotes However, its molecular mechanism is better understood in prokaryotes © McGraw Hill 81 Nucleotide Excision Repair Removes Damaged DNA Segments 2 In E. coli, the NER system requires four key proteins These are designated UvrA, UvrB, UvrC and UvrD • Named as such because they are involved in Ultraviolet light repair of pyrimidine dimers • They are also important in repairing chemically damaged DNA UvrA, B, C, and D recognize and remove a short segment of damaged DNA DNA polymerase and ligase finish the repair job © McGraw Hill 82 Figure 19.18 © McGraw Hill 83 Nucleotide Excision Repair Removes Damaged DNA Segments 3 Several human diseases have been shown to involve inherited defects in genes involved in NER • These include xeroderma pigmentosum (XP), Cockayne syndrome (CS) and PIBIDS • A common characteristic of all three syndromes is an increased sensitivity to sunlight • XP can be caused by defects in seven different NER genes © McGraw Hill 84 Figure 19.19 © McGraw Hill © Anne Chadwick Williams/Sacramento Bee/ZUMAPRESS.com/Newscom 85 Mismatch Repair Systems Recognize and Correct a Base Pair Mismatch 1 A base mismatch is another type of abnormality in DNA The structure of the DNA double helix obeys the AT/GC rule of base pairing • However, during DNA replication an incorrect base may be added to the growing strand by mistake DNA polymerases have a 3’ to 5’ proofreading ability that can detect base mismatches and fix them If proofreading fails, the mismatch repair system comes to the rescue © McGraw Hill 86 Mismatch Repair Systems Recognize and Correct a Base Pair Mismatch 2 Mismatch repair systems are found in all species An important aspect of these systems is that they are specific to the newly made strand Molecular mechanism of mismatch repair in E. coli • Three proteins, MutL, MutH and MutS detect the mismatch and direct its removal from the newly made strand • The proteins are named Mut because their absence leads to a much higher mutation rate than normal © McGraw Hill 87 Mismatch Repair Systems Recognize and Correct a Base Pair Mismatch 3 A key characteristic of MutH is that it can distinguish between the parental strand and the daughter strand • Prior to replication, both parental strands are methylated • Immediately after replication, the parental strand is methylated whereas the newly made daughter strand is not © McGraw Hill 88 Mismatch Repair The MutS protein slides along the DNA and finds a mismatch. The MutS/MutL complex binds to MutH, which is already bound to a hemimethylated sequence. Access the text alternative for slide images. © McGraw Hill Fig. 19.20 89 Double-Strand Breaks in DNA Can Be Repaired by Recombination DNA double-strand breaks are very dangerous • Breakage of chromosomes into pieces • Caused by ionizing radiation and chemical mutagens • Also caused by reactive oxygen species which are the by-products of cellular metabolism • 10 to 100 breaks occur each day in a typical human cell • Breaks can cause chromosomal rearrangements and deficiencies • They may be repaired by two systems known as homologous recombination repair (HRR) and nonhomologous end joining (NHEJ) © McGraw Hill 90 Repair of a Double-Strand Break by Homologous Recombination 1 A double-strand break is processed by the short digestion of the DNA strands Sister chromatids are only available during S and G2 of cell cycle • Used for strand exchange • Rarely, HRR can occur between non-identical chromosomes © McGraw Hill 91 Repair of a Double-Strand Break by Homologous Recombination 2 The unbroken strands are used as templates to synthesize DNA Strands are then broken and then rejoined in a way that produces separate chromatids • Because sister chromatids are genetically identical, homologous recombination can be an error-free repair mechanism © McGraw Hill 92 Figure 19.21 (1) Access the text alternative for slide images. © McGraw Hill 93 Figure 19.21 (2) © McGraw Hill 94 Non-Homologous End Joining Broken ends are recognized by end-binding proteins • Formation of crossbridge Processing may result in deletion of a small region • Not error free © McGraw Hill 95 Figure 19.22 Access the text alternative for slide images. © McGraw Hill 96 Damaged DNA May Be Replicated by Translesion DNA Polymerases 1 It is inevitable that some lesions may escape all repair systems • Such lesions may be present when DNA is replicated Replicative DNA polymerases, such as DNA pol III in E. coli, are sensitive to geometric distortions in DNA • They are unable to replicate through DNA lesions • This type of replication requires specialized DNA polymerases © McGraw Hill 97 Damaged DNA May Be Replicated by Translesion DNA Polymerases 2 Specialized enzymes assist the replicative DNA polymerase in the translesion synthesis (TLS) process The translesion-replicating polymerases contain an active site with a loose, flexible pocket • They can accommodate aberrant structures in the template strand • A negative consequence of translesion-replicating polymerases is their low fidelity • The mutation rate is typically in the range of 10-2 to 10-3 • Much higher than 10-8 of replicative polymerase © McGraw Hill 98 Damaged DNA May Be Replicated by Translesion DNA Polymerases 3 When a replicative DNA polymerase encounters a damaged region, it is swapped for a TLS polymerase • Region is duplicated with error-prone replication © McGraw Hill 99 19.6 Homologous Recombination Homologous recombination involves crossing over between identical or homologous regions of chromosomes • It occurs in meiosis I and occasionally during mitosis • Involves the alignment of a pair of homologous chromosomes, followed by breakage at analogous locations and exchange of corresponding segments Crossing over that occurs between sister chromatids is called sister chromatid exchange (SCE) • Sister chromatids are genetically identical to each other • SCE does not produce a new combination of alleles Crossing over that occurs between homologous chromosomes may produce new combinations of alleles © McGraw Hill 100 Figure 19.23 (a) Sister chromatid exchange (b) Recombination between homologous chromosomes during meiosis Access the text alternative for slide images. © McGraw Hill 101 The Holliday Model for Homologous Recombination The Holliday model can account for the general properties of homologous recombination during meiosis Deduced from genetic crosses in fungi A particularly convincing piece of evidence came from electron micrographs of recombination structures • The structure has been called a chi (χ) form © McGraw Hill 102 Figure 19.24b (b) Micrograph of a Holiday junction From: H. Potter and D. Dressler, “DNA Recombination: In Vivo and In Vitro Studies,” Cold Spring Harb Symp Quant Biol 1979.43: 969-985, © Cold Spring Harbor Laboratory Press. Image provided by Huntington Potter, Ph.D. © McGraw Hill 103 Figure 19.24a (1) Access the text alternative for slide images. © McGraw Hill 104 Figure 19.24a (2) (a) The Holliday model for homologous recombination Access the text alternative for slide images. © McGraw Hill 105 More Recent Models Have Refined the Steps of Recombination More detailed studies of genetic recombination have led to a refinement of the Holliday model In particular, more recent models have modified the initiation phase of recombination • Two nicks in the same location on two strands is unlikely • Rather, it is more likely for a DNA helix to incur a break in both strands of one chromatid • A double-strand break model was proposed by Jack Szostak, Terry Orr-Weaver, Rodney Rothstein and Franklin Stahl • Requires DNA gap repair synthesis © McGraw Hill 106 Figure 19.25 (1) Access the text alternative for slide images. © McGraw Hill 107 Figure 19.25 (2) © McGraw Hill 108 Various Proteins Facilitate Homologous Recombination 1 Molecular studies in two different yeast species suggest that double-strand breaks initiate the homologous recombination that occurs in meiosis In other words, double-strand breaks create sites where a crossover will occur In Saccharomyces cerevisiae, formation of DNA doublestrand breaks requires at least 10 different proteins • One of them, Spo11, is instrumental in actually breaking the DNA © McGraw Hill 109 Various Proteins Facilitate Homologous Recombination 2 Homologous recombination is found in all species • The cells of any given species may have more than one molecular mechanism for homologous recombination The enzymology of homologous recombination is best understood in E. coli • Table 19.8 summarizes some of the proteins involved • Note: The term Rec indicates that the proteins function in recombination © McGraw Hill 110 Table 19.8 E. coli Proteins that play a role in Homologous Recombination Protein Description RecBCD A complex of three proteins that tracks along the DNA and recognizes double-strand breaks. The complex partially degrades the double-stranded regions to generate single stranded regions that can participate in strand invasion. RecBCD is also involved in loading RecA onto single stranded DNA. In addition, RecBCD can create single-strand breaks that are used to initiate homologous recombination. Single-strand binding protein Coats broken ends of chromosomes and prevents excessive strand degradation. RecA Binds to single-stranded DNA and promotes strand invasion, which enables homologous strands to find each other. It also promotes the displacement of the complementary strand to generate a Dloop. RuvABC This protein complex binds to Holliday junctions. RuvAB promotes branch migration. RuvC is an endonuclease that cuts the crossed or uncrossed strands to resolve Holliday junctions into separate chromosomes. RecG RecG protein can also promote branch migration of Holliday junctions. © McGraw Hill 111 Causes of Gene Conversion Homologous recombination can cause two different alleles to become identical alleles • This process, whereby one of the alleles is converted to the other, has been termed gene conversion • The converted allele is close to the crossover site Gene conversion can occur in one of two ways 1. DNA mismatch repair • Refer to Figure 19.26 2. DNA gap repair synthesis • Refer to Figure 19.27 © McGraw Hill 112 Figure 19.26 © McGraw Hill 113 Gap Repair Synthesis 1 Gene conversion by gap repair synthesis according to the double-strand break model The top chromosome carries the recessive b allele, and the bottom chromosome carries the dominant B allele Figure 19.27 © McGraw Hill 114 Gap Repair Synthesis 2 Both chromosomes carry the B allele. © McGraw Hill Figure 19.27 115