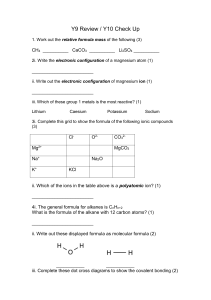
Lab – Determining the Enthalpy Change of a Reaction The reaction of hydrochloric acid with magnesium metal is represented by the following equation: 2HCl(aq) + Mg(s) MgCl2(aq) + H2(g) QUESTION: What is the enthalpy change (∆H) for the reaction between hydrochloric acid magnesium metal? SAFETY PRECAUTIONS: Aprons and safety glasses must be worn If you get hydrochloric acid on your skin or in your eyes, flush your skin or eyes with cold water for 15 minutes for eyes or 5 minutes for skin. MATERIALS: 1.00 mol/L HCl (aq) – corrosive and poisonous magnesium strip styrofoam cups (2) thermometer stir rod 100mL graduated cylinder 250mL beaker stop watch or timing device weigh scale PROCEDURE: 1. Build a calorimeter out of the three Styrofoam cups as demonstrated in class. You will need two holes in the top to put your stir rod and thermometer. The holes should be as small as possible to minimize thermal energy exchange with the surroundings. 2. Measure 50.0mL of HCl(aq) with the graduate cylinder and add the HCl(aq) to the calorimeter. 3. Record the initial temperature of the HCl(aq). 4. Measure and record the mass of the magnesium strip. Quickly and carefully add the magnesium strip to the 50.0mL of HCl(aq) in the calorimeter. 5. Place the top of the calorimeter in place and record the temperature every 30s. Stir gently and continuously. Ensure that the thermometer is not touching the bottom of the calorimeter. 6. When the temperature levels off, record the final temperature, tf. The magnesium strip should be completely dissolved. If the magnesium metal is not completely dissolved, wash off the remaining metal strip, allow it to dry and weigh the remaining metal strip. 7. Repeat steps 4-7 two more times for a total of three trials. OBSERVATIONS: Trail #1 Initial Temperature of HCl(aq):___________ Time (min. : sec.) 0:30 1:00 1:30 2:00 2:30 3:00 3:30 4:00 4:30 2:30 3:00 3:30 4:00 4:30 2:30 3:00 3:30 4:00 4:30 Temperature (oC) tf = _________________________ Trail #2 Initial Temperature of HCl(aq):___________ Time (min. : sec.) 0:30 1:00 1:30 2:00 Temperature (oC) tf = _________________________ Trail #3 Initial Temperature of HCl(aq):___________ Time (min. : sec.) 0:30 1:00 1:30 Temperature (oC) tf = _________________________ 2:00 ANALYSIS: 1. Is the reaction of dissolving magnesium metal endothermic or exothermic? Explain. 2. Determine the molar enthalpy change (∆rH) for the magnesium metal that dissolved for each trail. Show only one sample calculation for trail 1and then simply record your calculated values for trial 2 and 3. 3. Calculate the average molar enthalpy change for the reaction of magnesium metal. 4. Write a thermochemical equation for the reaction. (Show the actual calculation for how you go from a molar enthalpy change in question 3 to what I am asking in this question. 5. Based off of your thermochemical equation, what is the molar enthalpy change for HCl? 6. If the magnesium metal didn’t completely dissolve, why is it necessary to weigh the remaining amount of magnesium metal left? If a student forgot to weigh the remaining amount of magnesium and just used the total mass of the magnesium in the calculations, how would the measured enthalpy and molar enthalpy change differ from the correct values (ie. increase, decease, remain the same)? 7. Suppose you added the same amount of magnesium metal, but instead the magnesium was granular (cut up into small pieces) instead of one strip. Would you have obtained a different enthalpy change? Explain.