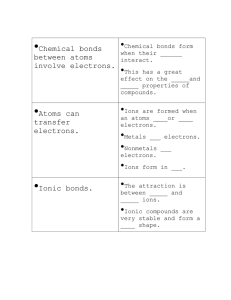
16 Ionic Bonding Some atoms are keen on getting rid of some of their electrons. Others want more. That’s life. And ions... Simple Ions Form When Atoms Lose or Gain Electrons | | | | | || || | | | | | | 1) Ions are charged particles — they can be single atoms (e.g. Na+) or groups of atoms (e.g. NO3–). 2) When atoms lose or gain electrons to form ions, all they’re trying to do is get a full outer shell (also called a “stable electronic structure”). Atoms with full outer shells are very stable. 3) Negative ions (anions) form when atoms gain electrons — they have more electrons than protons. Positive ions (cations) form when atoms lose electrons — they have more protons than electrons. || | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | || 4) The number of electrons lost or gained is the same as You calculate the number of protons and neutrons in the charge on the ion. E.g. If 2 electrons are lost the an ion in the same way as for an atom (see page 6). charge is 2+. If 3 electrons are gained the charge is 3–. | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | You Can Predict the Ions Formed From the Group Number 1) Group 1, 2 and 3 elements are metals. They lose electrons to form positive ions. Group 7 elements form 1– ions. 2) Group 5, 6 and 7 elements are non-metals. He H Group 1 elements They gain electrons to form negative ions. C N O B F Ne Li Be Group 3 elements form 1+ ions. form 3+ ions. Na Mg Al Si P S Cl Ar 3) Elements in the same group all have the Fe K Ca Sc V Cr Mn Co Ni Cu Zn Ga Ge As Se Br Kr Ti same number of outer electrons. So they Group 2 elements Ag Tc Ru Sr Y Zr Mo In Sn Sb Te I Xe Rb Nb Cd Pd Rh form 2+ ions. have to lose or gain the same number to Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn get a full outer shell. And this means that Fr Ra Ac Rf Db Sg Bh Hs Mt Ds Rg they form ions with the same charge. Group 5 elements 4) You don’t have to remember what ions most elements form Group 6 elements form 3– ions. — nope, you just look at the periodic table. form 2– ions. 5) Here are some trickier ions that you just have to learn: Ag+ Cu2+ Fe2+ Fe3+ Pb2+ Zn2+ Hydrogen: H+ Hydroxide: OH− Ammonium: NH4+ Carbonate: CO32− Nitrate: NO3− Sulfate: SO42− Ionic Bonding — Transfer of Electrons 1) When a metal and a non-metal react together, such as when Group 1 metals react with Group 7 elements, the metal atom loses electrons to form a positive ion (cation) and the non-metal gains these electrons to form a negative ion (anion). 2) These oppositely charged ions are strongly attracted to one another by electrostatic attractions. This attraction is called an ionic bond. 3) The reaction of sodium and chlorine is a classic case of ionic bonding: The sodium atom gives up its outer electron and becomes an Na+ ion. Na Cl The chlorine atom picks up the spare electron and becomes a Cl – ion. I’ve got my ion you... Don’t forget about the ions in the green box above. You can’t use the periodic table to work out the charges on these, like you can with the elements in Groups 1-3 and 5-7. You just need to learn them. Q1 State the charge on the following ions: a) ammonium, NH4 b) sulfate, SO4 c) nitrate, NO3 [3 marks] Q2 Describe, in terms of electron transfer, how sodium and chlorine react to form sodium chloride. Section 2 — The Periodic Table and Bonding [3 marks]