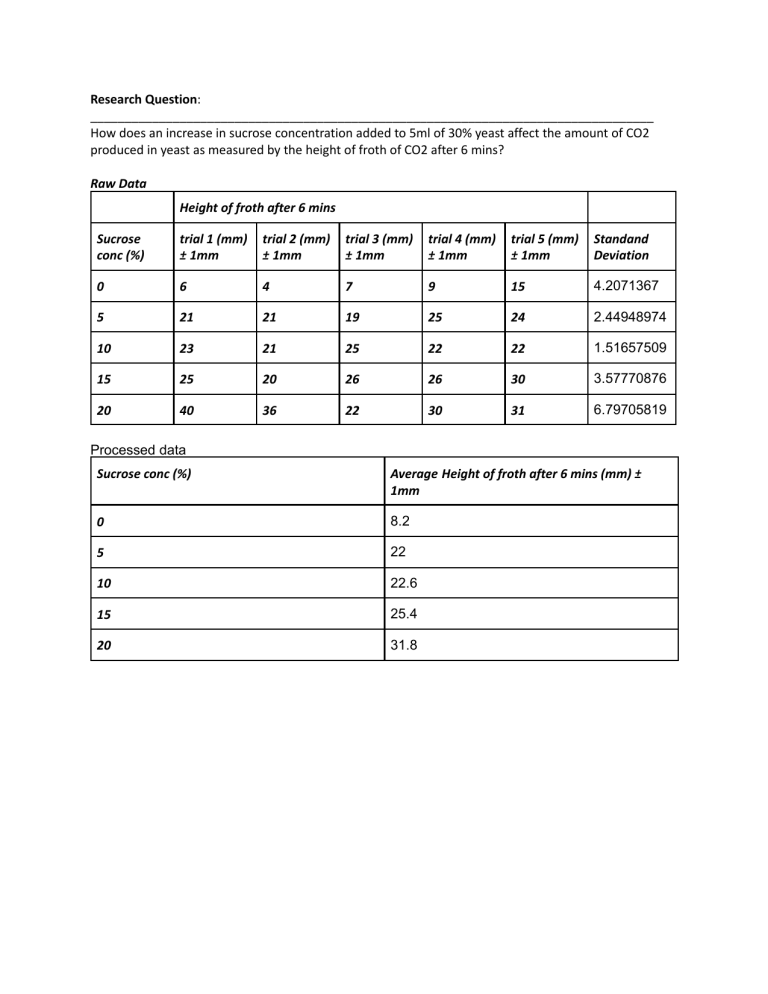
Research Question: __________________________________________________________________________________ How does an increase in sucrose concentration added to 5ml of 30% yeast affect the amount of CO2 produced in yeast as measured by the height of froth of CO2 after 6 mins? Raw Data Height of froth after 6 mins Sucrose conc (%) trial 1 (mm) ± 1mm trial 2 (mm) ± 1mm trial 3 (mm) ± 1mm trial 4 (mm) ± 1mm trial 5 (mm) ± 1mm Standand Deviation 0 6 4 7 9 15 4.2071367 5 21 21 19 25 24 2.44948974 10 23 21 25 22 22 1.51657509 15 25 20 26 26 30 3.57770876 20 40 36 22 30 31 6.79705819 Processed data Sucrose conc (%) Average Height of froth after 6 mins (mm) ± 1mm 0 8.2 5 22 10 22.6 15 25.4 20 31.8 Pearson Coefficient calculated by Excel (r): 0.92629881 → indicates a strong correlation Critical value for this experiment: 0.878 As r > 0.878 the results are statistically significant. As seen by the graph, the height of the yeast and sucrose concentration has a strong positive correlation. The Pearson coefficient for the data is 0.92629881, and the critical value for this experiment is 0.878 meaning it is statistically significant. Thus we can be more than 95% confident that the results of the experiment are not due to chance, and that if the experiment was repeated, similar results would be collected. Most of the data points are near the trendline with the exception of the 5% sucrose concentration. These data point seems to be the furthest away from the line. This is because there isn’t a large difference between the height of the froth measured at 5 and 10 % sucrose concentration. However, all the data points follow the trend since, as the sucrose concentration increase so do the heights of the froth. The error bars were calculated to show 1SD on either side of the mean. For some data points, the error bars are quite small like for 10 and 5 % sucrose concentrations, whereas the rest are quite large, the largest being 20% sucrose concentration. This means that for some of the variations there was a large spread of data whereas for some others there is a small spread of data. This suggests that some data collected for some variations were more reliable than others. This can be due to many reasons which will be talked about in the evaluation. In addition, there is quite a bit of overlap between the error bars, especially the last 4 data points. This suggests that data points collected for the different sucrose concentrations were similar for certain trials, which may be due to the limitations in the ‘evaluation’ In conclusion, as the sucrose concentration increases, so does the amount of CO2 produced by the yeast. Sucrose is utilized by the yeast through the process of respiration. In respiration, sucrose is broken down into glucose and fructose and glucose is used as a sugar source to produce carbon dioxide and water in the presence of oxygen. This process is called aerobic respiration and is given by the equation glucose + oxygen . The carbon dioxide produced in the yeat is seen in froth, hence increase in sucrose solution will produce a larger amount of glucose, which is used as a substrate for respiration. Therefore the rate of respiration increases, which increases the amount of carbon dioxide produced. This data supports the conclusion, as each data point follows this trend. For example, for sucrose concentrations 0, 5, 10, 15, 20, 25 % the heights of the froth are 8.2, 22, 22.6, 24.5, and 31.8 mm respectively. Additionally, most of the data points or at least their error bars interact with the trendline, with the exception of the 5% sucrose concentration. Strengths Significance: How it supported the methodology of the investigation Maintaining constant temperature Temperature was maintained at 40 degrees celsius using a water bath, and putting the samples in to acclimate. As this temperature in the optimal temperature for yeast. This is important as it allowed the reaction to occur quickly enough for the results to be collected, and made sure that the maltase in yeast did not denature, therefore the experiment could be carried out successfully. The fact that this was standardised for all samples in also important as it meant that temperature wasn’t affecting the rate of respiration, and the independent variable; sucrose concentration, was the only variable having an affect on the rate, thus making the results valid. Amount of trials per 5 trials were performed for each sucrose trial. Every experiment has some level sucrose of error. When an experiment is performed several times, various mistakes concentration tend to 'balance out,' and the outcome is statistically more accurate. Thus doing 5 trials per sucrose concentration is likely to have improved the accuracy of the final data. Limitation of the How significantly could this have method impacted on your results and why Improvement Carbon dioxide escaping As the test tubes were open and air was being allowed to escape, some carbon dioxide produced from the reaction may have escaped, whilst some got trapped in the solution. This would have resulted in a small height of froth recorded as the froth in the direct result of CO2 being trapped in the solution. This would have hindered the accuracy of the results, as the values recorded would have been lower than the actual amount of CO2 that would have been produced. This may also be the reason why the averages for 5% and 10% sucrose were so close, as CO2 may have escaped and caused a lower value for 10% sucrose to be measured. Although this would be a difficult limitation to improve, as the problem lies in the methodology of the experiment, a balloon can be places of the test tube so that CO2 doesnt not escape the the test tube. However, some CO2 may still escape from the solution and enter balloon, thus qualitative observations of the size of the balloon after 6 minutes a long with the measurement of froth can be use together to come to a final conclusion. Using sucrose Although sucrose can be used as a substrate in this experiment, it needs to first be broken down into fructose and glucose, and then only the glucose is used in respiration. This means that there is an unknown amount of time that it takes for the sucrose the break down into it’s monosaccharides. Thus it is difficult to know whether the time given to the samples to respire is long enough for the results to be accurate. Use varying concentrations of glucose solutions so that the sucrose doesn’t need to break down into glucose. Putting in and taking out the samples before measuring Before putting in the sample, the yeast was pipetted into the test tube with the sucrose. This was dones with 5 test tubes with the same concentration of sucrose. There was an inevitable delay caused by human error that resulted in some yeast being in contact with sucrose longer than others. Thus there was a slight difference between the amount of time each sample was undergoing respiration for. A similar circumstance occurred when they were being taken out of the water bath, as some samples froth was measured before some others. This added even more to the time difference and may have affected the results. This may have been the reason for the large error bars, as samples with the same concentration of sucrose showed a lot of variance. With more time, each trial can be performed one by one to limit the amount of time. Alternatively, a marking system can be used to mark the order in which the yeast was added to the test tubes, and the test tubes should be taken out of the water bath in the same order to all samples are reacting for approximately the same time. Extension: Further exploration can be done into how varying temperatures affect the rate of respiration of yeast, whilst controlling the sucrose concentration. The temperature could be easily manipulated using the water bath and setting the temperature at different intervals to explore its affects on respiration.


