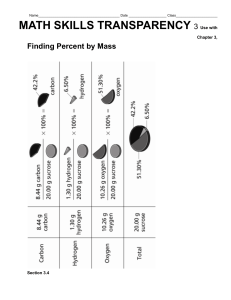
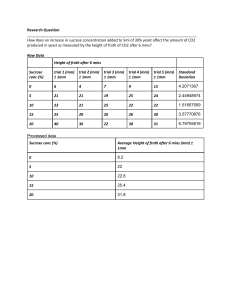
Solved problem-1: Estimate Theoretical Oxygen Demand (ThOD) for the following three following samples: a. Sample containing 500 mg/L of sucrose (C12H22O11) a. ThOD of the sample containing sucrose Stoicheometric equation for the oxidation of sucrose is C12H22O11 + 12O2 12CO2 + 11H2O 12 moles (364 grams) of oxygen is required for oxidizing one mole (342 grams) of sucrose Sucrose concentration of the sample = 500 mg/L ThOD of the sample = 500 mg / l X 364 grams 532 mg / L 342 grams