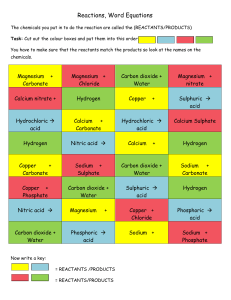
Name: Grade : 8 Chemistry Batch: Topic: Making Salts Method 1: Metal + acid salt + hydrogen Magnesium + sulphuric acid magnesium sulphate + hydrogen Complete the following equations: a) Zinc + Hydrochloric acid ……………. + ………………… b) Aluminium + Sulphuric acid ……………………+…………….. c) Iron + Hydrochloric acid ………………………..+…………….. Method 2: Metal oxide + acid salt + water Magnesium oxide + Hydrochloric acid magnesium chloride + water Complete the following equations: a) Zinc oxide + Hydrochloric acid ……………………. + ……………. b) Copper oxide + Sulphuric acid ……………………+…………….. c) Iron oxide + Nitric acid ………………………..+…………….. Method 3: Metal carbonate + acid salt + water + carbon dioxide Magnesium carbonate + hydrochloric acid magnesium chloride + water + carbon dioxide Complete the following equations: a) Copper carbonate + hydrochloric acid ----------------- +………………+……………………….. b) Zinc carbonate + sulphuric acid ……………. + ……………….. +…………………… Method 1: Metal + Acid 1. Measure out 10cm3 of dilute hydrochloric acid using a measuring cylinder. 2. Pour the acid into a beaker. 3. Add a small piece of magnesium ribbon to the acid in the beaker. 4. Continue adding magnesium until there are no more bubbles. 5. Filter the mixture. This removes the solid magnesium. 6. Pour the magnesium chloride solution into an evaporating dish. Heat the solution. When half of the water has evaporated, stop heating. Keep the evaporating dish at room temperature. Method 2: Metal carbonate + Acid 1. Measure out 10cm3 of dilute hydrochloric acid using a measuring cylinder. 2. Pour the acid into a beaker. 3. Add one spatula measure of copper carbonate powder. Carbon dioxide gas forms, and the reacting mixture bubbles. 4. Continue adding copper carbonate till no more bubbles form. The reaction has finished. 5. Filter the mixture to remove solid copper carbonate from the mixture. 6. Heat the copper sulphate mixture in an evaporating dish. Place it for a few days. Blue copper sulphate crystals can be seen.