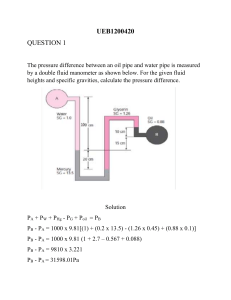
PROGRAM NATIONAL DIPLOMA CHEMICAL ENGINEERING SUBJECT CHEMICAL ENGINEERING TECHNOLOGY IIIA CODE CMTA321 DATE SUMMER EXAMINATION NOVEMBER 2007 MODEL ANSWERS QUESTION 1 1.1. 1.2. 1.6. 1.3. If one of the reactants is in excess, it will build up in the plant as there is no opportunity for it to leave. 1.4. Nitrogen in = nitrogen out 1.5. Mass flow-rate in = mass flow-rate out as required. QUESTION 2 2.1. Energy for 1kg of fluid: 2.2. Consider 1kg of fluid moving from point 1 to point 2: Assume T = constant, i.e. U 1 = U2 For incompressible flow, ρ = constant QUESTION 3 3.1. 3.2. Use the compressible gas equation for isothermal flow: Re>2100 therefore the flow is turbulent. Need to get f from the friction factor graph. 3.3. 3.4. Modes: Convection from air to pipe outer surface. Conduction through pipe. Convection from inner pipe wall to air. QUESTION 4 4.1. 4.2. 4.3.