Particle Nature of Matter Worksheet - Middle School Science
advertisement

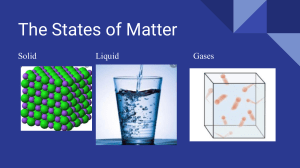
QUARTER 3: MODULE 1-2 THE PARTICLE NATURE OF MATTER Score: WW 3.1 - _______ / ________ Name: ___________________________________ Grade & Section: ______________ PT 3.1 - ________ / ________ Science Teacher: ___________________________ Date: _______________________ WRITTEN WORK 3.1 Multiple Choice. Choose the letter of the correct answer. Write your answer on the space provided before the number. ___________ 1. Which statement best explains the fact that gases can be compressed? A. The particles in a gas do not have a fixed volume. B. The particles in a gas are very far apart. C. The particles in a gas have no force of attraction (bonds) between them. D. The particles in a gas are constantly moving very rapidly. ___________ 2. Which of the following states of matter has the highest density? A. solid B. liquid C. gas D. plasma ___________ 3. In which state of matter are particles held together by weak forces of attraction and are able to move freely over long distance? A. solid B. liquid C. gas D. plasma ___________ 4. Which description best matches this particle model? A. Closely packed and vibrate about a fixed position. B. Particles are close together but can slide past each other. C. Particles have lots of energy and can move around freely D. Closely packed and slide past each other in a bounded container ___________ 5. Which statement about liquids is correct? A. They have a fixed shape and a fixed volume. B. They have a fixed shape but not a fixed volume. C. They have no fixed shape but they do have a fixed volume. D. They have no fixed shape and no fixed volume. ___________ 6. These particles are held together by very strong forces of attraction. A. solid B. liquid C. gas D. plasma ___________ 7. Arrange the strength of the forces of attraction between the particles of solids, liquids and gases from the strongest to weakest? A. (strongest) gases > liquids > solids (weakest) B. (strongest) solids > liquids > gases (weakest) C. (strongest) gases > solids > liquids (weakest) D. (strongest) solids > gases > liquids (weakest) ___________ 8. It is a state of matter that has no definite shape and volume. A. solid B. liquid C. gas D. plasma ___________ 9. Water boils at what temperature? A. 100 degrees Celsius B. 0 degrees Celsius C. 50 degrees Celsius D. 100 degrees Fahrenheit ___________ 10. Ice melts at what temperature? A. 100 degrees Celsius B. 0 degrees Celsius C. 50 degrees Celsius D. 100 degrees Fahrenheit PERFORMANCE TASK 3.1 TITLE: THE PARTICULATE NATURE OF MATTER (50 points) DIRECTIONS: Complete the table creatively and comprehensively. Compare the properties of SOLID, LIQUID, GAS. Use long bond paper following the uniform format with boarders. Make your work presentable. PROPERTIES SHAPE VOLUME DENSITY COMPRESSABILITY INTERMOLECULAR SPACES INTERMOLECULAR FORCE OF ATTRACTION ARRANGEMENT OF MOLECULES/DIAGRAM EXAMPLE CRITERIA FOR GRADING Content & Presentation – 25% Creativity & Lay-out – 10% Cleanliness & Organization – 10% Timeliness – 5% TOTAL: --------------------------- 50 SOLID LIQUID GAS