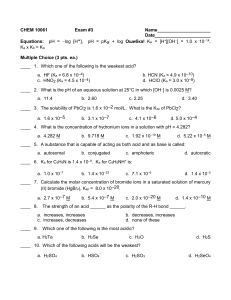
1 Experiment 7 Buffers and pH An important characteristic of water solutions is the concentration of hydronium ions (and also hydroxide ions) in the solution. The concentration of hydronium ions in water will (1) in some cases, determine the rate at which reactions occur in water, (a) For instance, the production of hydrogen from reaction of zinc metal with HCl solutions occurs more rapidly at higher hydronium ion concentration. (b) In the experiment to determine the equilibrium constant (Exp. # 6) HCl is used as a catalyst. (2) sometimes determine the actual direction in which a reaction occurs (which is equivalent to affecting the position of equilibrium of the reaction, which it should since it is a reactant). Oxidizing agents such as dichromate and permanganate will oxidize compounds (are better oxidizers) in acid solution (higher hydronium ion concentration) that they will not oxidize in basic solutions (lower hydronium ion concentration). Hydrogen peroxide will react with Mn +2 ion in basic solution to produce MnO2 (a precipitate). In acid solution, the reaction actually happens in reverse and hydrogen peroxide will react with MnO2 to produce Mn+2. Electrode potentials (see Experiment 9) can help in giving more information about which reactions occur (not the rate of reaction, however). Of course, in all the above cases, the hydronium ion takes part in the reaction process in some way. The concentration of hydronium ion is an intensive variable as are all concentrations. The pH (see below) is therefore an intensive variable. A commonly used method of indicating the concentration of hydronium ion is give the pH of the solution. The pH is defined as the negative logarithm of the hydronium ion concentration (molarity). (1) pH = -log [H3O+] Thus if [H3O+] = 1.4 x 10-3, then pH = -log (1.4 x 10-3) = -(-2.85) = 2.85. The pH of water solutions usually range from about 0 to 14 although the pH can be less than 0 or greater than 14. The pH does not give more information than the numerical value of the hydronium ion concentration about a solution but in some circumstances the use of pH is more convenient.. Solutions with pH = 7 (the pH of pure water) are said to be neutral; solutions with pH less than 7 are acidic and those with pH greater than 7 are basic. The lower the pH the greater the concentration of hydronium ion and the higher the pH the lower the concentration of hydronium ion. Because the relationship (2) [H3O+][OH-] = 1 x 10-14 2 must hold for water solutions, the concentration of hydroxide ions in the solution are greater at higher pH and smaller at lower pH. In many cases it is convenient to have a solution whose pH will change slowly on addition of acid or base. In biochemical and biological systems this stabilization of pH is often vitally important. The pH of blood has a normal range as given below: (3) men: women 7.27 to 7.43 7.31 to 7.43 Average = 7.35 Average = 7.37 The normal ingestion of food, etc., puts acids and bases in the blood (as well as other compounds) and it is important that mechanisms exist to prevent the pH of the blood from changing out of the above ranges. pH values of a person=s blood that are much below (acidosis) or above (alkalosis) the above values may be associated with serious, maybe even lethal, matabolic disorders in the body. A buffer solution is a solution whose pH changes slightly on the addition of an acid or a base. Buffer solutions are said to resist efforts to change their pH (the pH changes slightly) but they cannot prevent the change. The ingredients in a buffer are: 1. A mixture of a weak acid and the salt of the weak acid with a strong base. An equivalent way of saying this is that a buffer is a mixture of a weak acid and its conjugate base. Examples: (a) HOAc (the weak acid acetic acid) and NaOAc (or KOAc), the salt of the weak acid with a strong base (NaOH or KOH). Since the salt ionizes in water, this is essentially a mixture of HOAc and OAc-. OAc- is the conjugate base of the weak acid HOAc. (b) HNO2(the weak acid nitrous acid) and NaNO2 (or KNO2), the salt of the weak acid with a strong base (NaOH or KOH). Here, the NO2-(the nitrite ion) is the conjugate base of the weak acid HNO2. 2. A weak base and the salt of the weak base with a strong acid. Equivalently, a buffer solution contains a weak base and the conjugate acid of the weak base. Example: NH3 (the weak base) and NH4Cl (or NH4NO3), the salt of the weak base with a strong acid (HCl or HNO3). Here, NH4+ is the conjugate acid of the weak base NH3. A GENERAL STATEMENT OF THE INGREDIENTS OF A BUFFER IS THAT IT CONTAINS A 3 WEAK ACID OR BASE AND ITS CONJUGATE BASE OR ACID. MIXTURES THAT ARE NOT BUFFERS: HCl and NH4Cl (because HCl is a strong acid); NaOH and NaOAc (because NaOH is a strong base); HOAc and NH4Cl (because the salt is not the salt of the weak acid). THE AMOUNTS (CONCENTRATIONS) OF THE INGREDIENTS IN A BUFFER SOLUTION WILL AFFECT THE CAPACITY OF THE BUFFER, THAT IS, THE AMOUNT OF ACID OR BASE THAT CAN BE ADDED AND STILL HAVE THE BUFFER SOLUTION FUNCTION SUCH THAT THE pH DOES NOT CHANGE DRASTICALLY UPON ADDITION OF THE ACID OR BASE. THE LESS THE pH OF A SOLUTION CHANGES ON THE ADDITION OF A GIVEN AMOUNT OF ACID OR BASE, THE GREATER ITS BUFFER CAPACITY. THE AMOUNT (CONCENTRATION OR MOLES) OF THE INGREDIENTS IN A BUFFER SOLUTION MUST BE ALARGE@ RELATIVE TO THE AMOUNT (FINAL CONCENTRATION IN SOLUTION OR MOLES) OF ACID OR BASE THAT IS ADDED IF THE BUFFERING ACTION IS TO BE AEFFECTIVE@ (FOR THE pH TO CHANGE ONLY SLIGHTLY). I. BUFFER SOLUTION WITH THE CONCENTRATION OF BOTH COMPONENTS ALARGE@ RELATIVE TO THE AMOUNT OF ACID OR BASE ADDED Consider a (buffer) solution which is 0.2M HOAc and also 0.25M NaOAc. In this solution the equilibria below are both satisfied: (4) HOAc + H2O H3O+ + OAc- (5) OAc- + HOAc + OH- H2O Ka Kb = = 1.8 x 10-5 1 x 10-14 1.8 x 10-5 = 5.56 x 10-10 The pH of this solution can be calculated as follows from eq 4: (6) Ka = 1.8 x 10-5 = [H3O+][OAc-] [HOAc] = [H3O+](0.25) (0.2) [H3O+] = 1.44 x 10-5M (7) pH = 4.84 or from eq 5 as follows: (8) Kb = 5.56 x 10-10 = [HOAc][OH-] [OAc-] (9) [OH-] = 6.94 x 10-10M (10) (11) pOH = -log(6.94 x 10-10) = 9.16 pH = 14 - pOH = 4.84 = (0.2)[OH-] (0.25) In this solution the ratio of the concentration of acetate ion to acetic acid is (0.25/0.2) = 1.25 4 Consider 150 mL of the solution. In this volume there are (12) (13) (0.2M)(150) = 30 mmoles of HOAc (0.25)(150) = 37.5 mmoles of OAc- To 150 mL of this solution add 10.0 mL of a 0.1M solution of HCl. (14) (10.0 mL)(0.1M) = 1 mmole of HCl and therefore 1 mmole of H3O+ in the 10.00 mL. (15) In the 150 mL of solution, 1 mmole of OAc- is converted into 1 mmole of HOAc the conjugate base is converted into the acid by the addition of the HCl). ( After the addition, the total volume is 160 mL and the concentration of the HOAc and OAc- are (16) [HOAc] = 30 mmoles + 1 mmole 160 mL (17) [OAc-] 37.5 mmoles - 1 mmole 160 = = 0.194 M = 0.228 M The [H3O+] is then from eq 6 (18) 1.8 x 10-5 = [H3O+](0.228) (0.194) (19) [H3O+] = 1.53 x 10-5M (20) pH = 4.81 In this solution the ratio of the concentration of acetate ion to acetic acid is (0.228/0.194) = 1.175. Note that this ratio changed only from 1.25 to 1.175 which is the reason that the pH changed only slightly. The pH can be calculated using eq 8 and the same result as eq 20 will be obtained. Either of the equilibria can be used for this type of calculation. It should be noted that the pH changes only very slightly in this case. This is because the amount of acid added was small (1 mmole) relative to the amount of weak acid (30 mmoles) and conjugate base (37.5 mmoles). It should be noted that the pH did decrease because an acid was added to the solution. Suppose that 10.0 mL of 0.1 M NaOH is added to 150 mL of the acetic acid/sodium acetate solution. In this case (21) (22) (10.0)(0.1) = 1 mmole of NaOH = 1 mmole of OH- are added. 1 mmole of HOAc is converted to 1 mmole of OAc- in the solution. The concentrations of HOAc and OAc- after addition are 5 (23) [HOAc] (24) [OAc-] = = 30 mmoles - 1 mmole 160 mL 37.5 mmoles + 1 mmole 160 mL = 0.181 M = 0.241 M and the hydronium concentration and pH are by eq 6 (25) [H3O+] = (1.8 x 10-5)(0.181) (0.241) (26) pH = 4.87 = 1.35 x 10-5 The use of eq 8 will give the same result. The pH increased here because a base is added but the increase is small because of the buffering action of the solution and the mmoles of base added is small compared to the mmoles of buffer ingredients. The ratio of acetate ion concentration to acetic acid concentration has changed from 1.25 to 1.33 II. BUFFER SOLUTION IN WHICH THE CONCENTRATION OF ONE COMPONENT IS NOT ALARGE@ RELATIVE TO THE AMOUNT OF ACID OR BASE ADDED Consider 150 mL of a solution that is 0.2 M HOAc and 0.01M NaOAc. In this solution the ratio of the concentration of acetic acid to acetate ion is (0.2/0.01) = 20. The hydronium concentration of this solution is by eq 6 (27) (28) [H3O+] = (1.8 x 10-5)(0.2) 0.01 pH = 3.44 = 3.6 x 10-4M Note that the [HOAc] = 0.2 M here as it did in Part I above. The pH of the solution here is 3.44 as compared to 4.84 in Part I because of the difference in [OAc-]. There is more acetate ion in Part I above and the pH is higher (more basic). The acetate ion functions as a base (that is one reason it is called the conjugate base of HOAc). In the 150 mL of solution there are 30 mmoles of HOAc and 1.5 mmoles of OAcAdd 10 mL of 0.1 M HCl (1 mmole of HCl) to this solution. (29) 1 mmole of H3O+ is added to the solution and 1 mmole of OAc- is converted to 1 mmole HOAc The concentrations of HOAc and OAc- are then (30) [HOAc] = 30 mmoles + 1 mmole 160 mL = 0.194 M 6 (31) [OAc-] = 1.5 mmoles - 1.0 mmole 160 mL = 0.0031 M and the hydronium concentration is (32) (33) [H3O+] = pH = (1.8 x 10-5)(0.194) 0.0031 = 1.12 x 10-3 2.95 In this case the change in pH is 3.44 - 2.95 = 0.49 pH units. In this case the pH change is much larger than in part I (eqs 7 and 20 and 26 above) because the concentration of the sodium acetate in this case was not large relative to the amount of acid added. The ratio of the concentration of acetate ion to acetic acid is 63 after addition of the HCl (up from the original 20). Now add 10 mL of 0.1 M NaOH (1 mmole of NaOH) to 150 mL of the same solution.. (34) (10)(0.1) = 1 mmole of OH- is added and 1 mmole of HOAc is converted to 1 mmole of OAc - At that point (35) [HOAc] = 30 mmoles - 1 mmole 160 mL = 0.181 M (36) [OAc-] = 1.5 mmole + 1 mmole 160 mL = 0.0156 M The hydronium ion concentration and the pH are then (37) [H3O+] = (38) pH = 3.68 (1.8 x 10-5)(0.181) (0.0156) = 2.088 x 10-4 The solution is more resistant to pH changes upon the addition of base than addition of acid. However, in both cases for the 0.01 M sodium acetate solution the change in pH is greater than for the 0.25 M sodium acetate solution (0.2 M HOAc in both). The Acapacity@ (see below) of the buffer solution is less for the lower concentration of ingredients. THE ALARGER@ THE AMOUNT OF THE INGREDIENTS OF THE BUFFER RELATIVE TO THE AMOUNT OF ACID OR BASE ADDED THE BETTER THE BUFFER FUNCTIONS (THE HIGHER THE CAPACITY OF THE BUFFER). RATIONALE OF THE ABOVE CHANGES If one defines 7 (39) R = [HOAc] [OAc-] then eq 6 above can be solved for [H3O+] to give (a day without algebra is a day without sunshine): (40) [H3O+] = (1.8 x 10-5)(R) The hydronium ion concentration will not change much if the ratio R does not change much. For case I above for acid addition (41) Rinitial = (0.20)/(0.25) = 0.8 see eq 8 (42) Rfinal = (0.194)/(0.228) = 0.85 see eq 18 The ratio R does not change much and so the hydronium ion concentration does not change much. For case II above for acid addition (43) Rinitial = (0.2)/(0.01) = 20 see eq 27 (44) Rfinal = (0.194)/(0.0031) = 63 see eq 31 In this case the change in R is much larger and the pH changed much more than in case I. The difference is that the number of millimoles of acetate ion was small in case II and when some of it was converted to HOAc, the molarity of acetate ion changed a great deal. III. CAPACITY OF A BUFFER If the capacity of a buffer is defined as the millimoles of acid or base per liter of buffer needed to change the pH by one unit then (45) capacity = mmoles of acid or base added (V)(change in pH occurring) For part I above this gives (for either the acid or the base addition since the change in pH is the same for both) (46) capacity = (1 mmole) (0.15 L)(0.03 pH unit) = 222 mmoles/liter per pH unit For part II above 1. addition of acid (47) (48) capacity = (1 mmole) (0.15 L)(0.49 pH units) 2. addition of base capacity = (1 mmole) = 13.62 mmoles/liter per pH unit = 27.8 mmoles/liter per pH unit. 8 (0.15 L)(0.24 pH unit) It is then more apparent that the solution with the larger concentrations of weak acid and conjugate base has a larger capacity for resisting the addition of acid or base. IV. PREPARATION OF A BUFFER SOLUTION WITH A GIVEN pH Observation of the mass action expression in eq 6 indicates that if one has a fixed ratio of the concentrations of OAc- to HOAc, then the pH of the solution is fixed. Suppose one wished to prepare a solution whose pH = 4.95 and a 0.2 M solution of NaOAc and a 0.25 M solution of HOAc were available. If these two solutions are mixed in a given ratio, then [OAc -]/[HOAc] will have a value such that the [H3O+] will have to be the value that gives a pH of 4.95. If pH = 4.95, then (49) [H3O+] = 10-4.95 = (100.05)(10-5) = 1.12 x 10-5 M Using eq 6 and 49, one can write (50) 1.8 x 10-5 = (51) [OAc-] [HOAc] (1.12 x 10-5)[OAc-] [HOAc] = (1.8 x 10-5)/(1.12 x 10-5) = (1.8)/(1.12) = 1.60 A solution with a ratio of the concentrations of acetate ion to acetic acid of 1.60 will have pH = 4.95. If one wishes to prepare 100 mL of the mixture, then if Vs is the volume of sodium acetate solution and Va is the volume of acetic acid solution, and additive volumes are assumed, (52) mmoles HOAc added = (0.25M)(Va) (53) mmoles OAc- added = (0.2 M)(Vs) = (0.2 M)(100 - Va) In the final mixture of 100 mL, the concentrations are (54) [HOAc] = (55) [OAc-] = (0.25)(Va) 100 (0.2)(100 - Va) 100 Eqs 54 and 55 are substituted into eq 51 to give (56) (0.2)(100 - Va)/(100) (0.25)(Va)/(100) Eq 56 can be rearranged to give (56A) = (0.2)(100 - Va) (0.25)(Va) (0.2)(100 - Va) = (0.25)(Va)(1.60) = 1.60 9 (56B) 20 - 0.2Va = 0.4Va (56C) 0.6Va = 20 Solution of eq 56C for Va gives (57) Va = 33.33 mL = volume of 0.25 M acetic acid needed to make 100 mL of buffer with pH = 4.95 (58) Vs = 100 - 33.33 = 66.67 mL = volume of 0.20 M sodium acetate needed to make 100 mL of buffer with pH = 4.95 Therefore if 33.33 mL of 0.25 M HOAc solution is mixed with 66.67 mL of 0.20 M NaOAc solution, one will get 100 mL of a solution with a pH = 4.95. V. DILUTION OF A BUFFER SOLUTION Consider 50 mL of a 0.2 M HOAc solution which is also 0.25 M NaOAc. See part I above, eqs 4 to 11. Dilute this solution until the total volume is 100 mL. What is the pH of the diluted solution? For dilution, the equation MiVi = MfVf relates the initial volume and molarity (subscript i) to the final volume and molarity (subscript f). The concentration of HOAc and NaOAc in the diluted solution are (59) [HOAc]dil = (0.2)(50)/(100) = 0.1M (60) [OAc-]dil = (0.25)(50)/(100) = 0.125 M Eqs 59 and 60 are substituted into eq 6 to give (61) 1.8 x 10-5 = [H3O+](0.125) (0.1) (62) (63) [H3O+] = 1.44 x 10-5 M pH = 4.84 Comparison of eq 7 and eq 63 shows that diluting the buffer solution does not change the pH. The pH is controlled by the ratio of the concentrations of acetate ion to acetic acid. Diluting the solution changes both concentrations but by the same factor so that their ratio remains the same. If their ratio remains the same the hydronium ion concentration and the pH remain the same. 10 EXPERIMENTAL PROCEDURE 1. Your instructor will assign a pH value to each group. Calculate the exact volumes of the given solutions of HC2H3O2 (acetic acid, HOAc) and NaC2H3O2 (sodium acetate, NaOAc) needed to make 100 mL of a buffer solution having the pH assigned to you by your instructor. 2. Rinse and fill one buret with the provided HOAc solution. Rinse and fill a second buret with the provided NaOAc solution. Accurately measure the volumes calculated in step 1 above into a clean beaker and thoroughly mix. Inadequate mixing is one the most serious sources of error. The beaker need not be dry. A little distilled water (dilution, see discussion above) will not appreciably affect the pH of the buffer solution. 3. Prepare the following solutions in small beakers. Mark the beakers so they can be distinguished from each other. Measuring volumes using pipettes is probably easiest (STIR EACH WELL): (a). distilled water (the measured pH will be slightly less than 7)a (b) 25 mL of distilled water + 1 mL of 1M HCl (35 drops from a dropper bottle) (c) 25 mL of distilled water + 1 mL of 1M NaOH (35 drops from a dropper bottle) (d) 25 mL of your buffer soln + 1 mL of 1M HCl (35 drops from a dropper bottle) (e) 25 mL of your buffer soln + 1 mL of 1M NaOH (35 drops from a dropper bottle) (f) 25 mL of the sodium acetate solution used to prepare the buffer. The expected pH can be calculated. (g) 25 mL of the HOAc solution used to prepare the buffer. The expected pH can be calculated. (h) 25 mL or the remainder of the prepared buffer solution. The volumes of HCl and NaOH do not have to be exactly 1 mL but the two HCl volumes in (b) and (d) should be the same and the two NaOH volumes in (c) and (e) should be the same. A 1.0 mL pipette can be used here but using a counted number of drops ;may be more convenient. Counting drops is more accurate and precise than using a graduated cylinder. The number of drops to get approximately a milliliter varies with the dropper bottle but the above number is reasonable for the plastic dropper bottles currently in use. 4. Take these 8 solutions to the pH meter in the beakers they are prepared in and measure the pH of each of them. Rinse the pH electrode (probe) with distilled water (using a wash bottle) before using it for the first solution and before placing it in each solution to measure the pH of that solution. Rinse when finished and return to the storage solution beaker. The pH obtained for the buffer solution (and possibly for some others) may be somewhat lower than the value assigned (even if no volume errors are made) by up to about 0.1 pH unit due to the fact that the solution contains Na+ and C2H3O2- ions whose electric charge (indicated by the ionic strength of the solution) affects the behavior of the hydronium ion in solution. Compare the change in pH when HCl is added to distilled water (part b and part a) to the change in pH when HCl is added to the buffer solution (part d and h). Make the same comparison for the addition of NaOH to distilled water and the buffer solution. Calculate the capacity of the buffer. aThe pH of distilled water is less than 7 partly due to the absorption of CO 2 (a weak acid in water) into the water from air. Also the electrical difference in the buffer used to calibrate the pH meter and the solution whose pH is measured leads to an Aerror@ in the reading of the pH meter. REPORT: FOLLOW THE DIRECTIONS OF YOUR INSTRUCTOR.