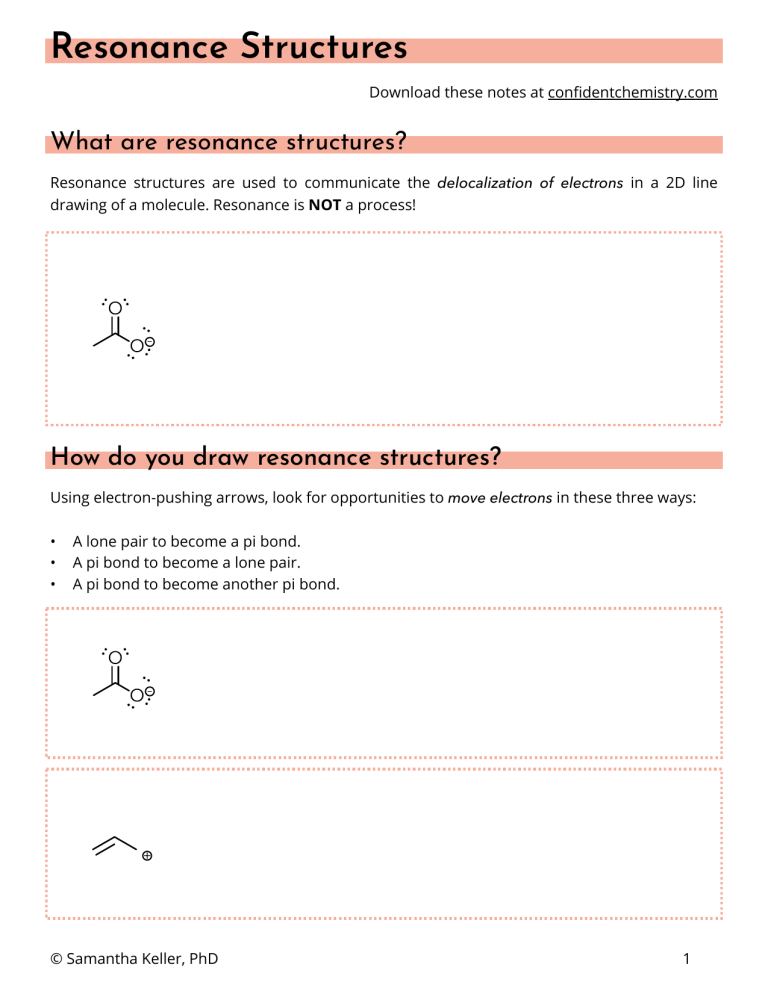
Resonance Structures Download these notes at confidentchemistry.com What are resonance structures? Resonance structures are used to communicate the delocalization of electrons in a 2D line drawing of a molecule. Resonance is NOT a process! How do you draw resonance structures? Using electron-pushing arrows, look for opportunities to move electrons in these three ways: • • • A lone pair to become a pi bond. A pi bond to become a lone pair. A pi bond to become another pi bond. © Samantha Keller, PhD 1 Non-equivalent resonance structures Each resonance structure is a “contributor” to the reality of the molecule. In some molecules, all of the contributors are equal. In other molecules, there are major contributors and minor contributors. The reality is like a weighted average. Sometimes, you are asked to rank or compare resonance contributors. Consider these guidelines when deciding which structure is a major or minor contributor: 1. Better resonance structures have more bonds and don’t violate the octet rule. 2. Better resonance structures have fewer formal charges. 3. Better resonance structures distribute charge according to electronegativity. © Samantha Keller, PhD 2 Practice Problems Draw all possible resonance structures for the following molecules? Which structures contribute most significantly to the “hybrid” or the reality of the molecule? What do the resonance structures tell us about how the molecule might react? © Samantha Keller, PhD 3


