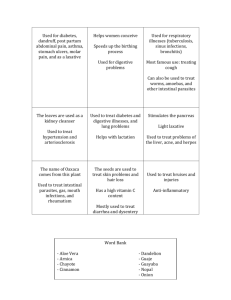
Journal of Critical Care 52 (2019) 86–91 Contents lists available at ScienceDirect Journal of Critical Care journal homepage: www.journals.elsevier.com/journal-of-critical-care The hospital-based evaluation of laxative prophylaxis in ICU (HELP-ICU): A pilot cluster-crossover randomized clinical trial Tyler Hay a, Adam M. Deane b,c,⁎, Tom Rechnitzer c, Kate Fetterplace b,c, Rebecca Reilly c, Melissa Ankravs b,c, Michael Bailey d,e, Timothy Fazio b,f, James Anstey b,c, Rohit D’Costa c, Jeffrey J. Presneill b,c, Christopher M. MacIsaac b,c, Rinaldo Bellomo b,c,d,g a The University of Melbourne, Melbourne Medical School, Parkville, Victoria, Australia The University of Melbourne, Melbourne Medical School, Department of Medicine and Radiology, Royal Melbourne Hospital, Parkville, VIC 3050, Australia c Intensive Care Unit, The Royal Melbourne Hospital, Parkville, Victoria, Australia d Australian and New Zealand Intensive Care Research Centre, Monash University School of Public Health and Preventive Medicine, Melbourne, Australia e The University of Melbourne, Department of Medicine and Radiology, Parkville, Victoria, Australia f Business Intelligence Unit, The Royal Melbourne Hospital, Parkville, Victoria, Australia g Intensive Care Unit, The Austin Hospital, Heidelberg, Victoria, Australia b a r t i c l e i n f o a b s t r a c t Purpose: Prophylactic laxative regimens may prevent constipation but may increase diarrhea and subsequent rectal tube insertion. Our aim was to compare three prophylactic laxative regimens on the rate of rectal tube insertion (primary outcome) and major constipation- or diarrhea-associated complications. Material and Methods: We conducted a cluster-crossover trial. Three pods in a single ICU were each randomized to one of three regimens for four months with rolling cross-over. All mechanically-ventilated and enterally-fed adult patients received either regimen: A) one coloxyl with senna BD from day one; B) two coloxyl with senna +20 ml lactulose BD commencing on day 3; or C) two coloxyl with senna tablets +20 ml lactulose BD commencing on day 6. Results: We enrolled 570 patients (A = 170, B = 205, C = 195) with similar baseline features. Overall, 53 (9.3%) patients received a rectal tube, and insertion rate was not statistically different between the three regimens (A = 12.9%, B = 7.8%, C = 7.7%; p = 0.15). The proportions of patients with other major constipationor diarrhea-associated complications were similar, as were major patient-centred outcomes. Conclusion: Earlier commencement of a prophylactic coloxyl-based laxative regimen (day 1 or 3) did not affect the rates of complications associated with constipation or diarrhea when compared to delayed introduction (day 6). © 2019 Published by Elsevier Inc. Keywords: Constipation Diarrhea Critically ill Enteral nutrition Laxatives Rectal tube 1. Introduction Constipation describes either infrequent bowel movement or difficulty in passing stool [1,2]. In most critically ill patients, the symptom of difficulty passing stool can be challenging to determine due to sedation. Nonetheless, infrequent bowel movements (non-defecation) has been reported as a frequent condition in the critically ill, possibly due to abnormal gastrointestinal motility [3,4]. Symptoms associated with infrequent bowel movements, or constipation, are subjective and Abbreviations: MV, Mechanical ventilation; ICU, Intensive care unit; APACHE, Acute physiology and chronic health evaluation; ANZROD, The Australian and New Zealand Risk of Death; EOLC, End of life care; ECMO, Extra corporeal membrane oxygenation. ⁎ Corresponding author at: Department of Intensive Care, Royal Melbourne Hospital, 300 Grattan St, Parkville, Victoria, Australia. E-mail address: adam.deane@mh.org.au (A.M. Deane). include abdominal distension, nausea, vomiting, and restlessness [5,6]. Profound gastrointestinal dysmotility, however, can lead to intestinal pseudo-obstruction, which puts patients at risk of bowel perforation, both of which are associated with adverse patient-centered outcomes [7,8]. Thus, the use of prophylactic laxative regimens in enterally fed critically ill patients has been proposed as a method to prevent such adverse effects [9]. However, prophylactic laxative regimens risk the development of diarrhea, which is also a frequent condition in the critically ill [10,11]. Diarrhea may also cause harm due to malabsorption of nutrients, electrolyte imbalance, skin breakdown, dehydration, infection, need for patient isolation, expense with stool testing to exclude Clostridium difficile, and increased duration of intensive care unit (ICU) admission [12-15]. Further, diarrhea may compromise patient dignity and increase nursing workload [16,17]. Finally, troublesome diarrhea may require the insertion of a rectal tube for liquid stool management [18]. Whilst https://doi.org/10.1016/j.jcrc.2019.04.010 0883-9441/© 2019 Published by Elsevier Inc. Downloaded for Anonymous User (n/a) at Queensland Health Clinical Knowledge Network from ClinicalKey.com.au by Elsevier on November 19, 2019. For personal use only. No other uses without permission. Copyright ©2019. Elsevier Inc. All rights reserved. T. Hay et al. / Journal of Critical Care 52 (2019) 86–91 the impact of these devices on patient comfort and dignity has not been described in detail, insertion of these tubes can result in life-threatening complications [19,20]. In light of the above issues, we performed a trial to evaluate the effects of three prophylactic laxative regimens. We hypothesized that earlier administration of a laxative bowel regimen may reduce constipation-associated complications but increase diarrhea-associated complications in particular insertion of rectal tubes, when compared with a delayed laxative regimen. 2. Methods 2.1. Study design We conducted a prospective cluster-crossover randomized clinical trial in mechanically ventilated enterally fed critically ill patients admitted to a mixed medical-surgical Intensive Care Unit (ICU). Due to an absence of evidence and widespread variance in local practice, the Hospital Human Research and Ethics Committee (HREC) provided approval using a waiver of consent with all data collected as part of routine care and management. 87 occurred by day 5 an osmotic agent was commenced, one movicol sachet (macrogol 3350 13.125 g, sodium chloride 350.8 mg, sodium bicarbonate 178.6 mg, potassium chloride 50.2 mg) and continued daily. 2.4.2. Regimen B No laxatives were administered until day 3 of enteral nutrition, when two coloxyl with senna tablets and 20 ml lactulose were commenced and administered twice daily. 2.4.3. Regimen C No laxatives were administered until day 6 of enteral nutrition, when two coloxyl with senna tablets and 20 ml lactulose were commenced and administered twice daily. Patient assignment was based on the initial ICU pod they were admitted to. Pods were randomly allocated to each of the laxative bowel regimens for a four-month period. The study was commenced on 1 July 2017, and the last patient was enrolled on 30 June 2018. 2.5. Data collection Baseline demographic, diagnostic and illness severity data were collected for all patients. 2.2. Setting 2.6. Primary outcome Our ICU is comprised of three pods, each having between 10 and 12 beds, and all containing a mix of operative and non-operative patients, for a total of 32 beds within in a 571-bed quaternary referral teaching hospital. 2.3. Patients All mechanically ventilated, enterally fed patients ≥18 years of age admitted to the ICU were eligible to be enrolled. Exclusion criteria included: life expectancy b24 h; patients undergoing end of life or palliative care; patients already receiving aperients or laxatives upon admission to the ICU; patients with a condition causing established diarrhea or constipation on admission; patients with a primary gastrointestinal tract (GIT) disease, GIT pathology, or GIT surgery as cause for admission to the ICU; patients receiving lactulose to treat encephalopathy; patients admitted with a spinal cord injury; patients who required rectal tubes to be inserted as part of hospital protocol including those receiving extra-corporeal membrane oxygenation (ECMO), ventilation in the prone position, and those who had extensive plastic surgery and/or a muscle flap, extensive wounds, burns, or necrotising fasciitis; or previously participated. 2.4. Study protocol Commencement of enteral feeding was at the discretion of the treating clinician, but the ICU has a proactive approach to commencement of enteral feeding (Appendix 1) [21]. The commencement of enteral feed was considered as day 1 with a day being a calendar day (i.e. day 1 was a partial day). All patients remained in their original assigned regimen throughout their index ICU admission. The prophylactic laxative bowel regimen was continued daily unless the treating clinician deemed that it was in the best interests of the patient were to withhold the regimen on that day and/or the patient had three or more bowel actions in the previous 24-h period. The intervention was continued until enteral feeding was no longer required, the patient was discharged from ICU or the patient died. Rescue aperients or enemas were discouraged but could be administered by the treating clinician. All three regimens were delivered via a nasogastric tube, and were as follows: 2.4.1. Regimen A One coloxyl with senna tablet twice daily, starting the day that enteral nutrition was commenced and continued daily. If no bowel action We chose the insertion of a rectal tube as the primary outcome. This is because the insertion of a rectal tube is a marker of intractable diarrhea and having a rectal tube in situ is likely to be important to patients. Furthermore, as there is wide variation in taxonomy used to define diarrhea [22], using the insertion of a rectal tube as a proxy measurement also limits reporting bias. All rectal tubes were inserted by a trained senior nursing staff member. These data were prospectively collected by treating intensivist using paper Case Report Forms, which were cross referenced against electronic patient progress notes. 2.7. Secondary outcomes We selected hospital codes, using the International Classification of Disease version 10, Australian Modification (ICD-10 AM) codes of interest, for diarrhea, paralytic ileus, and intestinal obstruction (Appendix 2) as hospital coders provide an objective assessment of any of these complications and remain blinded to the intervention. Patients who received a rectal tube, yet were not coded for ‘diarrhea’, were assumed to have developed diarrhea and were included as such for analysis. We also selected the recording of pressure wounds in a clinical incident management system, RiskMan Pty Ltd. (Melbourne, Australia): ‘pressure wounds’ were restricted to the anatomical areas of sacrum, ischium, coccyx, or buttocks. 2.8. Tertiary outcomes We collected duration of mechanical ventilation as total number of hours and duration of ICU admission as total number of days. Mortality was recorded as 30-day mortality with all patients discharged alive from hospital assumed to be alive at day 30. 2.9. Data acquisition Our hospital has a mature data warehouse which stores data from a variety of clinical and administrative data sets. Data pertaining to patient complications were taken from two source systems: the patient administration system (i.Patient Manager [i.PM APAC] version 10.1.7 Computer Sciences Corporation) and RiskMan (version 160,101.160224, RiskMan International Pty Ltd. [Australia]). Data linkage between datasets was performed using admission dates, discharge dates, incident reporting dates, and patient hospital record numbers. Downloaded for Anonymous User (n/a) at Queensland Health Clinical Knowledge Network from ClinicalKey.com.au by Elsevier on November 19, 2019. For personal use only. No other uses without permission. Copyright ©2019. Elsevier Inc. All rights reserved. 88 T. Hay et al. / Journal of Critical Care 52 (2019) 86–91 These data were interrogated using Structured Query Language (SQL) through SQL Server Management Studio (version 11.0.6020.0, Copyright 2012 Microsoft). Clinical coding data were retrieved for all patients who were admitted to the ICU between 1 July 2017 and 30 June 2018 and queried for International Classification of Disease version 10, Australian Modification (ICD-10 AM) codes of interest. Clinical incidents which listed the ICU as the location of occurrence and occurred between 1 July 2017 and 30 June 2018 were retrieved, and queried for pressure ulcers occurring at the sacrum, ischium, coccyx, or buttocks. The number of stool samples sent for each patient and positive Clostridium difficile culture data were obtained from the hospital pathology department. 2.10. Statistical analysis All data were initially assessed for normality. Group comparisons were performed using chi-square tests for equal proportion (or Fisher's exact tests where numbers were small), analysis of variance (ANOVA) for normally distributed data, and Kruskal Wallis tests otherwise, with results reported as n (%), mean (standard deviation), or median (interquartile range) respectively. Univariable and multivariable analyses adjusting for severity were performed using logistic regression with results presented as odds ratios (95% CI) referenced against regimen C with patient severity given by the Australian and New Zealand Risk of Death (ANZROD) [23]. Analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and a two-sided p-value of 0.05 was used to indicate statistical significance. In the absence of sufficient prior data, one of the principal aims of this pilot study was to inform sample size for future trials. As such, the sample size for this study was principally one of convenience, encompassing a 12-month enrolment period. 3. Results 3.1. Patients During the study period, 664 patients met all inclusion criteria with 94 meeting at least one exclusion criterion (Fig. 1). Of the 570 patients included, 170 received regimen A; 205 received regimen B; and 195 received regimen C. Baseline demographic data revealed no statistically significant differences between the three groups (Table 1). 3.2. Primary outcome In total, 53/570 (9.3%) patients had a rectal tube inserted. It appears that patients who had a rectal tube inserted had greater illness severity (Table 2). Twenty-two (12.9%) patients had a rectal tube when receiving regimen A, 16 (7.8%) while receiving regimen B, and 15 (7.7%) while receiving regimen C. There was no statistical difference between the three groups (p = 0.15). When analysed using raw and severity adjusted logistic regression, the results remained unchanged. Raw odds ratios (referenced against regimen C): regimen A 1.78 (95% CI, 0.89–3.56); p = 0.05, regimen B: 1.02 (95% CI, 0.49–2.12); p = 0.38. Severity adjusted odds ratios (referenced against regimen C): regimen A: 1.79 (95% CI, 0.90–3.57); p = 0.05, regimen B: 1.03 (95% CI, 0.49–2.15); p = 0.40). 3.3. Secondary outcomes There were no statistical differences in the development of diarrhea, paralytic ileus or intestinal obstruction, local pressure wounds, or stool samples sent for testing (Table 3). Only one of the 72 samples sent from 55 patients returned a positive Clostridium difficile result (1.3%). 3.4. Tertiary outcomes There was no difference in duration of mechanical ventilation between the three groups. Likewise, duration of ICU admission did not differ between groups. There was no significant difference in mortality between groups (Table 3), or time to death (Appendix 3). However, patients who had a rectal tube inserted represent a unique sub-population. This cohort of patients received prolonged periods of mechanical ventilation and ICU admission (Table 4). Patients who met primary inclusion criteria n = 664 Excluded patients (n=94) EOLC (n=30) Receiving lactulose therapy for liver disease (n=12) Primary GIT surgery (n=10) Primary GIT pathology (n=9) Admitted with spinal injury (n=8) Regimen not charted (n=8) Already on aperients (n=7) Patient on ECMO (n=3) Patient receiving prone ventilation protocol (n=2) Surgery/wound that requires flap, burn, necrotising fasciitis (n=1) Receiving trophic feeds (n=1) Patients meeting multiple exclusion criteria (n=3) Patients enrolled in study n = 570 Regimen A n = 170 Regimen B n = 205 Regimen C n = 195 Fig. 1. Study flow diagram for included patients. Abbreviations: EOLC end of life care, including palliative care and life expectancy b24 h; ECMO extra corporeal membrane oxygenation. Downloaded for Anonymous User (n/a) at Queensland Health Clinical Knowledge Network from ClinicalKey.com.au by Elsevier on November 19, 2019. For personal use only. No other uses without permission. Copyright ©2019. Elsevier Inc. All rights reserved. T. Hay et al. / Journal of Critical Care 52 (2019) 86–91 89 admission, or mortality between patients receiving each of the three regimens. Table 1 Baseline patient characteristics. Variable All patients n = 570 Regimen A n = 170 Regimen B n = 205 Regimen C n = 195 p value Male gender Age, years Admission category Non-operative Post-operative ICU cause of admission Trauma Neurological Cardiovascular Respiratory Sepsis Metabolic Gastrointestinal Other APACHE III, points ANZROD, % 385 (67.5) 113 (66.5) 144 (70.2) 128 (65.6) 0.58 57.1 ± 17.7 58.3 ± 16.5 57.0 ± 17.3 56.3 ± 19.1 0.57 0.74 400 (70.2) 170 (29.8) 123 (72.4) 47 (27.6) 143 (69.8) 62 (30.2) 134 (68.7) 61 (31.3) 175 (30.7) 120 (21.1) 112 (19.6) 70 (12.3) 40 (7.0) 25 (4.4) 14 (2.5) 14 (2.5) 70.4 ± 29.2 13.8 (3.7–35.7) 52 (30.6) 33 (19.4) 30 (17.6) 23 (13.5) 14 (8.2) 7 (4.1) 6 (3.5) 5 (2.9) 72.9 ± 29.1 16.0 (5.0–36.8) 66 (32.2) 38 (18.5) 46 (22.4) 23 (11.2) 11 (5.4) 10 (4.9) 5 (2.4) 6 (2.9) 66.6 ± 27.2 10.9 (3.6–30.7) 57 (29.2) 49 (25.1) 36 (18.5) 24 (12.3) 15 (7.7) 8 (4.1) 3 (1.5) 3 (1.5) 72.2 ± 30.9 0.07 15.0 0.15 (3.5–40.8) Comparisons performed using Chi-squared test, Fisher's exact test, ANOVA, and KruskalWallis test as appropriate. Results are expressed as number (%), mean ± standard deviation, or median (25th–75th percentile). ICU intensive care unit, APACHE III Acute Physiology and Chronic Health Evaluation III, ANZROD The Australian and New Zealand Risk of Death. 4. Discussion 4.1. Key findings In our pilot, cluster-crossover, randomized clinical trial of 570 mechanically ventilated, enterally fed, adult patients, we found that rectal tube insertion occurred in close to 10% of patients. However, we observed no difference in such use when comparing earlier versus delayed laxative regimens. Moreover, diarrhea or complications possibly associated with diarrhea or constipation occurred relatively frequently but at similar rates across the three study groups. Finally, we found no difference in the duration of mechanical ventilation, duration of ICU Table 2 Patient characteristics according to rectal tube insertion. Variable All patients n = 570 Rectal tube inserted n = 53 No rectal tube n = 517 p value Male gender Age, years Admission category Non-operative Post-operative ICU cause of admission Trauma Neurological Cardiovascular Respiratory Sepsis Metabolic Gastrointestinal Other APACHE III, points ANZROD, % 385 (67.5) 57.1 ± 17.7 33 (62.3) 56.3 ± 18.9 352 (68.1) 57.2 ± 17.6 0.39 0.72 0.57 400 (70.2) 170 (29.8) 39 (73.6) 14 (26.4) 361 (69.8) 156 (30.2) 175 (30.7) 120 (21.1) 112 (19.6) 70 (12.3) 40 (7.0) 25 (4.4) 14 (2.5) 14 (2.5) 66.5 (49–88) 13.8 (3.7–35.7) 17 (32.1) 9 (17) 9 (17) 6 (11.3) 5 (9.4) 4 (7.5) 1 (1.9) 2 (3.8) 80 (62–99) 21.4 (10.2–35.8) 158 (30.6) 111 (21.5) 103 (19.8) 64 (12.4) 35 (6.8) 21 (4.1) 13 (2.5) 12 (2.3) 65 (48–87) 13.3 (3.6–35.8) 0.003 0.06 Comparisons performed using Chi-squared test, Student t-test, Fisher's exact test, ANOVA, and Kruskal-Wallis test as appropriate. Results are expressed as number (%), mean ± standard deviation, or median (25th–75th percentile). ICU intensive care unit, APACHE III Acute Physiology and Chronic Health Evaluation III, ANZROD The Australian and New Zealand Risk of Death. 4.2. Relationship to previous studies A previous systematic review and meta-analysis identified a total of three prospective randomized clinical trials of prophylactic laxative regimens [8,24,25]. Taken together, previous trials have provided data from b500 patients. The point estimates from this meta-analysis suggest that prophylactic laxative regimens may reduce constipation and increase diarrhea, but the 95% confidence intervals for both outcomes crossed the midline. Our trial therefore randomized a greater number of patients than all previous trials to date. However, we could not identify any particular regimen as superior. All three of these previous trials implemented a lactulose-based prophylactic laxative bowel regimen, with one trial also implementing a third polyethylene glycol (PEG)-based treatment arm [8], which differed from the coloxyl with senna laxative ± lactulose regimens used in our trial. We opted for the use of coloxyl with senna due to clinician familiarity at our institution; their well-documented, and dual mechanisms of action, as both a stool softener and stimulant; and the scarcity of evidence in the literature to guide their use in the critically ill population. In previous trials of prophylactic laxative regimens, the proportion of patients who developed constipation was reported. We decided not to record constipation or lack of bowel actions per se because of the lack of a standardized definition for such outcomes in the critically ill, and because the implications for patients with non-defecation are unknown [26]. However, the development of paralytic ileus and intestinal obstruction was recorded, in essence capturing the most severe end of this spectrum, and that which is most strongly associated with adverse patient outcomes [7]. There exist data in the literature on the incidence of diarrhea in critically ill patients receiving nasogastric feeding, though the taxonomy used to define diarrhea is highly variable [10,11,13,14,22,27-31]. However, the insertion of a rectal tube implies diarrhea severe enough to warrant this invasive intervention. Based on this assumption, approximately 10% of our patients experienced severe diarrhea. No study has previously reported on the prevalence of rectal tube insertion in such patients and the outcomes of such a subpopulation of critically ill patients. Only 2.1% of patients developed a complication of non-defecation, either paralytic ileus or intestinal obstruction. Previous observational studies have reported a point prevalence of ‘constipation’ in critically ill patients anywhere between 20% and 83% [3,5]. This variation may partially be explained by the lack of consistent taxonomy used to define constipation, and the heterogeneity between study populations. The finding in our trial of 2.1% of patients with a complication of nondefecation is far less than previous studies describing prevalence of ‘constipation’. However, if the aim of prophylactic laxative regimens is to prevent complications from non-defecation, and effectiveness was deemed to halve such events, based on the point estimate from our trial the number needed to treat would be approximately 100. Although constipation is a frequently reported outcome in intensive care research trials, in ambulant patients constipation is identified on the basis of excessive straining, sense of incomplete evacuation, failed or lengthy attempts to defecate, hard stools, and, less frequently, by the number of stools per week [32]. Because many patients in the ICU cannot describe these symptoms, ‘non-defecation’ and ‘impaired gastrointestinal motility’ may be terms better suited to this population [25] and severe complications associated with non-defecation a more objective outcome to measure. 4.3. Study implications Our findings imply that in mechanically ventilated, enterally fed, critically ill patients, major complications associated with non- Downloaded for Anonymous User (n/a) at Queensland Health Clinical Knowledge Network from ClinicalKey.com.au by Elsevier on November 19, 2019. For personal use only. No other uses without permission. Copyright ©2019. Elsevier Inc. All rights reserved. 90 T. Hay et al. / Journal of Critical Care 52 (2019) 86–91 Table 3 Outcomes of patients according to prophylactic laxative regimen. Outcome Primary outcome Rectal tube insertion Secondary outcomes Diarrhea Paralytic ileus or intestinal obstruction Patients who had ≥1 stool sample sent for pathology Positive Clostridium difficile cultures Local pressure wound Tertiary outcomes Duration of mechanical ventilation, hours Duration of ICU admission, days 30-day mortality All patients n = 570 Regimen A n = 170 Regimen B n = 205 Regimen C n = 195 p value 53 (9.3) 22 (12.9) 16 (7.8) 15 (7.7) 0.15 78 (13.7) 13 (2.3) 55 (9.6) 1 18 (3.2) 25 (14.7) 4 (2.4) 20 (11.8) 0 7 (4.1) 21 (10.2) 2 (1.0) 14 (6.8) 1 4 (2.0) 29 (14.9) 7 (3.6) 21 (10.8) 0 7 (3.6) 0.30 0.22 0.22 0.41 0.45 81.4 (39.0–156.0) 4.9 (3.0–8.8) 108 (18.9) 94.0 (41.9–164.0) 5.7 (3.4–9.8) 30 (17.6) 78.0 (37.9–148.0) 4.9 (3.0–8.6) 34 (16.6) 76.4 (37.4–154.0) 4.5 (2.9–8.4) 44 (22.6) 0.34 0.17 0.27 Comparisons performed using Chi-square test, ANOVA, and Kruskal-Wallis test as appropriate. Results are expressed as number (%), or median (25th–75th percentile). ICU intensive care unit. defecation occur infrequently but that the use of rectal tubes occurs in approximately 10% of patients. Moreover, they imply that complications from excessive defecation associated with treatment to prevent nondefecation are sufficiently frequent to justify further studies aimed at optimising prophylactic laxative regimens in this population, including whether they should be used at all. Finally, our data suggest that delaying prophylactic laxative regimens until day six of ICU admission may be safe. 4.4. Strengths and limitations Our trial has several strengths. It was a prospective, clustercrossover randomized trial, and included the largest number of patients randomized to various laxative bowel regimens in the literature. Due to the study design, the risk of selection bias was minimized and individual selection bias attenuated. Furthermore, ascertainment bias was minimized as outcomes required an intervention (i.e. diarrhea leading to the insertion of a rectal tube) or were identified using hospital-coding data. Nonetheless, our trial has some important limitations. It is a singlecentre trial, including only mechanically ventilated, enterally fed patients. Bias may also have been introduced by the non-blinded design, particularly given our primary outcome (insertion of a rectal tube) was at the discretion of treating clinicians (including investigators). We did not have a control (i.e. no treatment), and so we cannot exclude the possibility that laxative bowel regimens cause greater harm than no treatment. However, prior to commencement of our trial, albeit it with wide inter-clinician variation, all treating intensivists at our institution believed that a prophylactic laxative bowel regimen should be commenced at some point during a patient's ICU admission. We are also limited to providing information for coloxyl with senna ± lactulose-based regimens, given at different times. We acknowledge that the optimal laxative bowel regimen may include none of these agents, with many hospitals in other regions moving away from the use of coloxyl with senna. Because we did not restrict inclusion in this trial to patients anticipated to stay for extended periods (e.g. five or more days), our data provides limited inferences when comparing early or delayed initiation of prophylactic bowel regimens in the subgroup of patients that are expected to have prolonged periods of mechanical ventilation and enteral feeding. Our trial was a pragmatic introduction of prophylactic laxative regimens complemented by a comprehensive education program, however compliance with was not measured. Finally, there is imprecision surrounding the secondary outcomes as event rates were obtained from the centralized hospital data and rely on accurate reporting in the patient notes and identification by hospital coders. However, these sources of imprecision should have equally affected patients treated with different regimens. Despite the limitations, the results of our trial provide substantial information to guide clinical practice and suggests that clinicians using prophylactic laxative regimens can commence such prophylaxis on day six without a major risk of non-defecation associated complications. Nonetheless, our observations are consistent with the concept that complications associated with impaired gastrointestinal motility are sufficiently frequent that further studies are warranted to identify optimal prophylactic laxative regimens to reduce complications. 5. Conclusion The administration of a delayed prophylactic laxative regimen (coloxyl with senna with or without lactulose) on day six after commencing enteral nutrition did not cause a significant difference in rectal tube insertion rates, or complications associated with either Table 4 Outcomes of patients who received a rectal tube. Outcome Secondary outcomes Paralytic ileus or intestinal obstruction Patients who had ≥1 stool sample sent for pathology Local pressure wound Tertiary outcomes Duration of mechanical ventilation, hours Duration of ICU admission, days 30-day mortality Time from ICU admission to death, days All patients n = 570 Rectal tube inserted n = 53 No rectal tube n = 517 p value 12 (2.1) 55 (9.6) 13 (2.3) 1 (1.9) 27 (50.9) 3 (5.7) 11 (2.1) 28 (5.4) 10 (1.9) 0.91 b0.0001 0.08 81.4 (39.0–156.0) 4.9 (3.0–8.8) 108 (18.9) 5.8 (3.3–11.8) 216.0 (116.0–332.0) 12.6 (8.0–21.2) 5 (9.4) 15.7 (12.6–67.5) 75.4 (37.4–138.0) 4.6 (2.9–7.7) 103 (19.9) 5.6 (3.1–10.0) b0.0001 b0.0001 0.06 b0.0001 Comparisons performed using Chi-squared test, Fisher's exact test, Wilcoxon rank-sum, ANOVA, and Kruskal-Wallis test as appropriate. Results are expressed as number (%), mean ± standard deviation, or median (25th–75th percentile). ICU intensive care unit, APACHE III Acute Physiology and Chronic Health Evaluation III, ANZROD The Australian and New Zealand Risk of Death. Downloaded for Anonymous User (n/a) at Queensland Health Clinical Knowledge Network from ClinicalKey.com.au by Elsevier on November 19, 2019. For personal use only. No other uses without permission. Copyright ©2019. Elsevier Inc. All rights reserved. T. Hay et al. / Journal of Critical Care 52 (2019) 86–91 constipation or diarrhea. However, rectal tube use was frequent and occurred in 10% of all patients. These findings suggest the need to conduct further studies to optimize prophylactic laxative regimens. Conflict of interest This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflict of interest. Acknowledgements We wish to thank Mr. Patrick McCrohan, The Royal Melbourne Hospital ICU data manager, and Ms. Maria Bisignano, from Melbourne Health shared pathology service, for their assistance in providing important patient data. Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.jcrc.2019.04.010. References [1] Hay T, Bellomo R, Rechnitzer T, See E, Ali Abdelhamid Y, Deane A. Constipation, diarrhea, and prophylactic laxative bowel regimens in the critically ill: A systematic review and meta-analysis. [Epub ahead of print] J Crit Care 2019 Jan 10. https://doi. org/10.1016/j.jcrc.2019.01.004 pii: S0883-9441(18)31259-0. [2] Fukuda S, Miyauchi T, Fujita M, Oda Y, Todani M, Kawamura Y, et al. Risk factors for late defecation and its association with the outcomes of critically ill patients: a retrospective observational study. J Intensive Care 2016;4:33. [3] Mostafa SM, Bhandari S, Ritchie G, Gratton N, Wenstone R. Constipation and its implications in the critically ill patient. Br J Anaesth 2003;91(6):815–9. [4] Deane AM, Chapman MJ, Reintam Blaser A, SA McClave, Emmanuel A. Pathophysiology and Treatment of Gastrointestinal Motility Disorders in the Acutely Ill. Nutr Clin Pract 2019 Feb;34(1):23–36. [5] Nguyen T, Frenette AJ, Johanson C, Maclean RD, Patel R, Simpson A, et al. Impaired gastrointestinal transit and its associated morbidity in the intensive care unit. J Crit Care 2013;28(4) (537 e11–7). [6] Patel PB, Brett SJ, O'Callaghan D, Anjum A, Cross M, Warwick J, et al. Protocol for a randomised control trial of methylnaltrexone for the treatment of opioid-induced constipation and gastrointestinal stasis in intensive care patients (MOTION). BMJ Open 2016;6(7):e011750. [7] Ross SW, Oommen B, Wormer BA, Walters AL, Augenstein VA, Heniford BT, et al. Acute colonic pseudo-obstruction: defining the epidemiology, treatment, and adverse outcomes of Ogilvie's syndrome. Am Surg 2016;82(2):102–11. [8] van der Spoel JI, Oudemans-van Straaten HM, Stoutenbeek CP, Bosman RJ, Zandstra DF. Neostigmine resolves critical illness-related colonic ileus in intensive care patients with multiple organ failure–a prospective, double-blind, placebo-controlled trial. Intensive Care Med 2001;27(5):822–7. [9] Oczkowski SJW, Duan EH, Groen A, Warren D, Cook DJ. The use of bowel protocols in critically ill adult patients: a systematic review and meta-analysis. Crit Care Med 2017;45(7):e718–26. [10] Jack L, Coyer F, Courtney M, Venkatesh B. Diarrhoea risk factors in enterally tube fed critically ill patients: a retrospective audit. Intensive Crit Care Nurs 2010;26(6): 327–34. 91 [11] Ozgur M, Girgin NK, Akalin H, Iscimen R, Sinirtas M, Kahveci F. A retrospective evaluation of the incidence and risk factors of nosocomial diarrhea in critically ill adult patients. Acta Medica Mediterr 2010;32(3):6. [12] de Brito-Ashurst I, Preiser JC. Diarrhea in critically ill patients: the role of enteral feeding. JPEN J Parenter Enter Nutr 2016;40(7):913–23. [13] Tirlapur N, Puthucheary ZA, Cooper JA, Sanders J, Coen PG, Moonesinghe SR, et al. Diarrhoea in the critically ill is common, associated with poor outcome, and rarely due to clostridium difficile. Sci Rep 2016;6:24691. [14] Reintam Blaser A, Deane AM, Fruhwald S. Diarrhoea in the critically ill. Curr Opin Crit Care 2015;21(2):142–53. [15] Jakob SM, Butikofer L, Berger D, Coslovsky M, Takala J. A randomized controlled pilot study to evaluate the effect of an enteral formulation designed to improve gastrointestinal tolerance in the critically ill patient-the SPIRIT trial. Crit Care 2017;21 (1):140. [16] Heidegger CP, Graf S, Perneger T, Genton L, Oshima T, Pichard C. The burden of diarrhea in the intensive care unit (ICU-BD). A survey and observational study of the caregivers' opinions and workload. Int J Nurs Stud 2016;59:163–8. [17] Smith CE, Faust-Wilson P, Lohr G, Kallenberger S, Marien L. A measure of distress reaction to diarrhea in ventilated tube-fed patients. Nurs Res 1992;41(5): 312–3. [18] Johnstone A. Evaluating flexi-seal® FMS: a faecal management system. Wounds UK 2005;1:5. [19] Padmanabhan A, Stern M, Wishin J, Mangino M, Richey K, DeSane M, et al. Clinical evaluation of a flexible fecal incontinence management system. Am J Crit Care 2007;16(4):384–93. [20] Daniel ES, Ng A, Johnston MJ, Ong EJ. Rectal bleeding post the use of the flexi-seal faecal management system. ANZ J Surg 2018;88(1–2):E83–4. [21] TARGET Investigators on behalf of the Australian and New Zealand Intensive Care Society Clinical Trials Group. Study protocol for the augmented versus routine approach to giving energy trial (TARGET). Crit Care Resusc 2018;20(1): 6–14. [22] Lebak KJ, Bliss DZ, Savik K, Patten-Marsh KM. What's new on defining diarrhea in tube-feeding studies? Clin Nurs Res 2003;12(2):174–204. [23] Paul E, Bailey M, Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand risk of death model. J Crit Care 2013;28(6): 935–41. [24] de Azevedo RP, Freitas FG, Ferreira EM, Pontes de Azevedo LC, Machado FR. Daily laxative therapy reduces organ dysfunction in mechanically ventilated patients: a phase II randomized controlled trial. Crit Care 2015;19:329. [25] Masri Y, Abubaker J, Ahmed R. Prophylactic use of laxative for constipation in critically ill patients. Ann Thorac Med 2010;5(4):228–31. [26] Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. recommendations of the ESICM working group on abdominal problems. Intensive Care Med 2012;38(3):384–94. [27] Bishop S, Young H, Goldsmith D, Buldock D, Chin M, Bellomo R. Bowel motions in critically ill patients: a pilot observational study. Crit Care Resusc 2010;12(3): 182–5. [28] Reintam A, Parm P, Kitus R, Kern H, Starkopf J. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand 2009;53(3):318–24. [29] Reintam Blaser A, Poeze M, Malbrain ML, Björck M, Oudemans-van Straaten HM, Starkopf J, et al. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med 2013;39(5):899–909. [30] Reintam Blaser A, Starkopf L, Deane AM, Poeze M, Starkopf J. Comparison of different definitions of feeding intolerance: a retrospective observational study. Clin Nutr 2015;34(5):956–61. [31] Thibault R, Graf S, Clerc A, Delieuvin N, Heidegger CP, Pichard C. Diarrhoea in the ICU: respective contribution of feeding and antibiotics. Crit Care 2013;17(4): R153. [32] Rao SS, Meduri K. What is necessary to diagnose constipation? Best Pract Res Clin Gastroenterol 2011;25(1):127–40. Downloaded for Anonymous User (n/a) at Queensland Health Clinical Knowledge Network from ClinicalKey.com.au by Elsevier on November 19, 2019. For personal use only. No other uses without permission. Copyright ©2019. Elsevier Inc. All rights reserved.