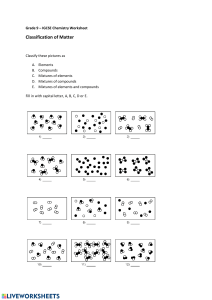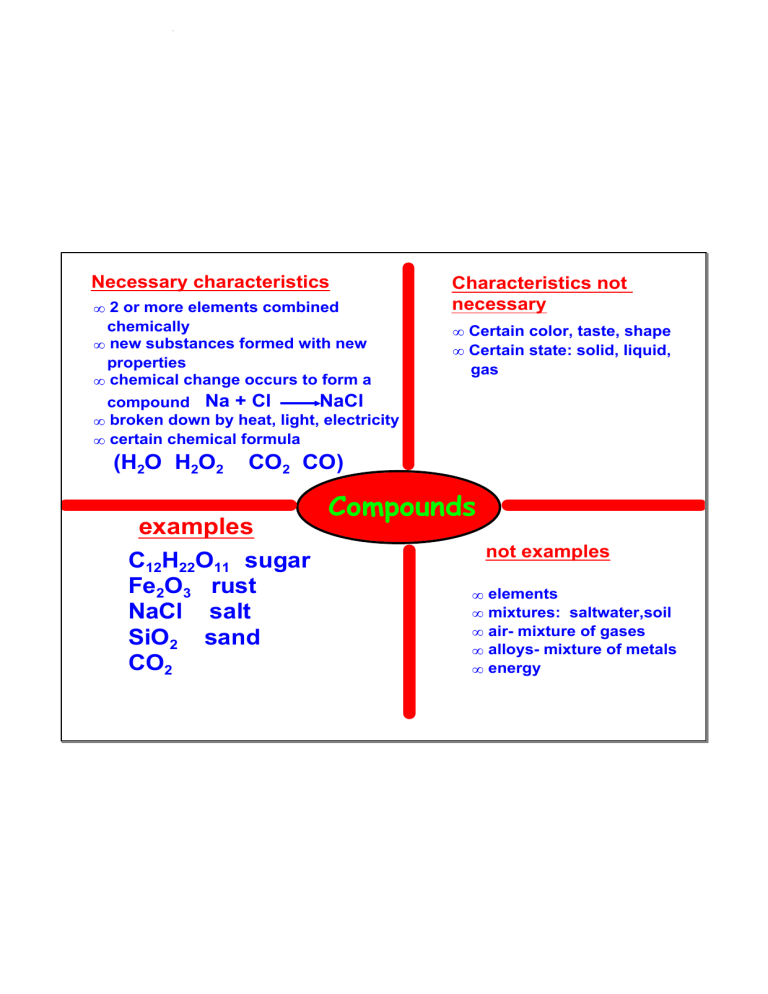
compounds and mixtures February 07, 2020 Necessary characteristics • 2 or more elements combined chemically • new substances formed with new properties • chemical change occurs to form a Characteristics not necessary • Certain color, taste, shape • Certain state: solid, liquid, gas NaCl compound Na + Cl • broken down by heat, light, electricity • certain chemical formula (H2O H2O2 CO2 CO) examples C12H22O11 sugar Fe2O3 rust NaCl salt SiO2 sand CO2 Compounds not examples • • • • • elements mixtures: saltwater,soil air­ mixture of gases alloys­ mixture of metals energy 1 compounds and mixtures February 07, 2020 Compound : 2 or more elements combined chemically that can be separated by heat, light, or electricity. Physical Ways of Separation filter, sort, strain, sift, dissolve, boil, condense, evaporate mixture : two or more substances combined physically, but not joined chemically, that can be separated physically mixture : any amount of each substance. Has no set proportion or ratio. compounds : have a certain formula H2O H2O2 CO2 CO 2