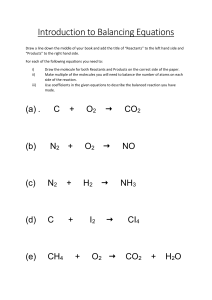
CHEMISTRY FOR ENGINEERS - LEC 2nd Semester Ay 2020 - 2021 CHEMICAL REACTION & STOICHIOMETRY Learning Outcomes: Define chemical reaction, chemical equation, coefficients, reactants and product. Identify the types of chemical reaction Write correctly the chemical formulas of reactants and products in a chemical reaction Balance a chemical equation Analyze and solve problems on the amount of reactants or products used/form in a chemical reaction Chemical Reaction A process that transform one or more substances into another set of substances Chemical changes Change in composition Example: Ethyl alcohol + Oxygen Lead (IV) oxide Gasoline + Oxygen Carbon dioxide + Water Lead (II) Oxide + Oxygen Carbon dioxide + water Law of Conservation of Mass In a chemical reaction: Mass before reaction Reactants Mass of reactants consumed Mass after reaction Products Mass of products produced Reactants – substances that are consumed in a reaction Products – substances that are produced or formed in a reaction Sample Problem: If 2 g of iron is heated with chlorine gas, 5.81 g of ferric chloride forms. How much chlorine was used up in the reaction? Iron + Chlorine Mass of Fe + Mass of Cl = Ferric Chloride Mass of FeCl 3 Mass of Cl = Mass of FeCl 3 – Mass of Fe = 5.81 g – 2g = 3.81 g Chemical Equation The symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side A chemical equation uses symbols and formula instead of the names of the elements and compounds participating in the reaction Ex. Sodium + Chlorine 2Na + Cl2 Sodium Chloride 2NaCl2 Notations Used in Chemical Equation 1. (g) – for gas 2. (l) – for liquid 3. (s) - for solid 4. (aq) – for aqueous 5. Δ - denotes heat Coefficient Is the number placed before the symbol or formula of the participating substances Represents the number of moles of reactants needed or product formed in the reaction. If no coefficient appears before a given formula, the number 1 is understood. N2 + 3 H2 Coefficients 2NH3 Writing and Balancing Chemical Equation 1. Identify the reactants and products. Look for some keywords. 2. Write the correct formula of the reactants and products 3. Balance the equation by trial and error using coefficients. Balance the atom that appears only once either in the reactants or in the products. The number of atoms in the reactants should be equal to the number of atoms in the products. 4. Indicate the states of matter of the reactants and products Consider this reaction: Water by electrolysis process produces hydrogen gas and oxygen gas: H2 O 2H2O(l) 2 mole(18g/mole) 36g 36g H2 + O2 2H2(g) + O2(g) 2moles(2g/mole) + 1 mole(32g/mole) 4 g + 32 g 36g Sample ? CH4 + ? O2 → ? CO2 + ?H2O CH4 + O2 → CO2 + H2O CH4 + O2 → CO2 + 2 H2O CH4 + 2 O2 → CO2 + 2 H2O CH4 + 2 O2 → CO2 + 2 H2O (balance equation) Balance these equation: 1.) Fe + O2 2.) KClO3 3.) Ca(OH)2 + H2SO4 Fe2O3 KCl + O2 H2O + CaSO4 Balance these equation 1.) 4 Fe + 3O2 2.) 2KClO3 3.) Ca(OH)2 + H2SO4 2Fe2O3 2KCl + 3O2 2H2O + CaSO4 Types of Chemical Reactions 1. Combination - occurs when two or more substances either elements or compounds react to form one product A + B Ex. C(s) + O2(g) → AB CO2(g) 2. Decomposition - occurs when one compound decompose into two or more new substances. AB Ex. 2H2O(l) → CaCO3(s) → A + B 2H2(g) + O2(g) CaO(s) + CO2(g) 3. Single Displacement or Single Substitution - a more active element takes the place of another element in a compound and sets the less active one free. • • A + BX → AX + B AX + Y → AY + X A free metal can generally displace a less active metal in a compound. A free nonmetal can generally displace a less active nonmetal in a compound. Ex. Fe(s) + CuSO4(aq) FeSO4(aq) + Cu(s) Cu(s) + FeSO4(s) N. R. (no reaction) 2Na(s) + 2H2O(l) → Zn(s) + 2HCl(aq) → 2NaOH(aq) + H2(g) ZnCl2(aq) + H2(g) ACTIVITY SERIES OF NON-METALS Most Active Least Active Example: F Cl Br I NaF + Cl2 → N. R. ACTIVITY SERIES OF METALS Most Active Li K Ba Ca Na Mg Al Mn Zn Cr Fe Cd Ni Sn Pb H Cu Hg Ag Au Least active 4. Double Substitution or Double Displacement - the reaction of two compounds in which two other compounds are produced, as a result of a trade of the anions by the cations. AB + CD → AD + CB Ex. NH4Cl(aq) + AgClO3(aq) NH4ClO3(aq) + AgCl(s) Ca(OH)2(s) + 2HNO3(aq) Ca(NO3)2(aq) + 2H2O(l) 5. Combustion - a process also known as “burning”. - rapid reaction of a wide variety of substances with oxygen gas. Ex. S(s) + O2(g) → SO2(g) C + O2(sufficient Amount) → CO2 C + O2(insufficient Amount) → CO Exercise: Balance the following chemical reaction and identify its type. Mg(OH)2 + 2HCl → MgCl2 + 2H2O P2O5 + 3H2O → 2 H3PO4 3(NH4)2S(aq) + 2FeCl3(aq) → Fe2S3 + 6NH4Cl Fe + 2 AgNO3 → Fe(NO3)2 + 2 Ag 2KClO3(s) → 2 KCl + 3O2 Exercise: Propane, C3H8, is used as a fuel for gas barbecue grills, where it burns in a controlled fashion. But if a mixture of propane is ignited in a closed space, like a gas pipeline an explosion can easily result. In either of these cases, the propane combines with oxygen O2 to form carbon dioxide and water. Write a balanced chemical equation describing this reaction. Solution: Identify the reactants and products. Look for keywords (italics). Write the correct formula and the chemical equation. Reactants on the left side of the equation while products on the right side of the equation. ANSWER: C3H8 + 5O2 → 3CO2 + 4H2O Mass Relations from Equations/Stoichiometry Given the following chemical equation: + N2O4 → 3N2 + 4H2O 2N2H4 This is read as: 2 mols N2H4 + 1 mol N2O4 → 3 mols N2 + 4 mols H2O The quantities 2 moles N2H4, 1 mol N2O4, 3 mols N2 and 4 mols H2O are chemically equivaleny to each other in this reaction. Hence they can be use in conversion factors such as: 2 𝑚𝑜𝑙𝑠 𝑁2 𝐻4 3 𝑚𝑜𝑙 𝑁2 ; 3 𝑚𝑜𝑙 𝑁2 1 𝑚𝑜𝑙 𝑁2 𝑂4 ; or a variety of other combinations These are called stoichiometric ratios. Use the balanced equations for the ratios. Problem: Ammonia is used to make fertilizers for lawns and gardens by reacting nitrogen gas with hydrogen gas. a.) Write a complete balanced chemical equation. b.) How many moles of ammonia are formed when 1.34 mol of nitrogen react? c.) How many grams of hydrogen are required to produced 2750 g of ammonia? Solution: a. b. N2 + 3H2 → 2NH3 nN2 = 1.34 mole nNH3 = 1.34 mols of N2 x 2 𝑚𝑜𝑙𝑒𝑠 𝑜𝑓 𝑁𝐻3 1 𝑚𝑜𝑙𝑠 𝑜𝑓 𝑁2 nNH3 = 2.68 mols c. Mass of NH3 = 2750 g , m of H2 = ? Stoichiometric ratios Solution: c. Mass of NH3 = 2750 g , m of H2 = ? Find no. of moles of NH3 = mass/molar mass = 2750g/17 g/mols = 161.76 moles nH2 = 161.76 moles NH3 x = 242.65 moles 3 𝑚𝑜𝑙𝑠 𝐻2 2 𝑚𝑜𝑙𝑠 𝑁𝐻3 mH2 = nH2 x molar mass = 242.65 moles x 2 g/moles = 485.3 g Problem Set: A. Balance the following chemical equations: 1. CaC2 + H2O → Ca(OH)2 + C2H2 2. (NH4)2Cr2O7 → Cr2O3 + N2 + H2O 3. CH4 + NH3 + O2 → HCN + H2O 4. SO2 + H2O → H2SO4 5. HNO3 + P4O10 → HPO3 + N2O5 6. Al + HCl → AlCl3 + H2 7. H2SO4 + NaOH → Na2SO4 + H2O Problem Set: B. 1. Answer the following problems Ethanol, C2H5OH , is responsible for the effects of intoxication felt after drinking alcoholic beverages. When ethanol burns in oxygen, carbon dioxide and water are produced. a. White a balanced chemical equation for the reaction b. How many liters of ethanol (density = 0.789 g/cm3 will be produced 1.25 L of water (density = 1.0 g/cm3) Problem Set: B. 2. Answer the following problems The combustion of liquid chloroethylene, C2H3Cl, yields carbon dioxide, steam and hydrogen chloride gas. a. Write a balanced chemical equation for the reaction. b. How many moles of oxygen are required to react with 35.00 g of chloroethylene? References Hill, John W. (2013). Chemistry for Changing Times. New York: Prentice Hall Masterton, William, et al. General Chemistry. Philippines: Cengage Learning Asia Pte Ltd Stoker, H. Stephen (2016). General Chemistry. Andover: Cengage Learning Asia Pte Ltd. Zumdahl, Steven S. (2015). Introductory Chemistry: Foundation. USA: Cengage Learning Asia Ptd Ltd., USA A