Chem 519 Practice Quiz: Ceramics, Superconductors, Polymers
advertisement

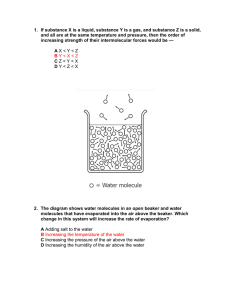
Chem 519 Vaibhav’s Practice Midterm Quiz 1. Which of the following properties belongs to Ceramics: A. Soft and weak B. Hard and strong C. Hard and brittle D. Hard and tough E. None of these 2. Which of the following is superconductor in nature? A. Graphite B. C60 C. C60 with alkali cations D. C60 without cations E. None of these 3. Name the small molecule compound that generates stacks that conduct an electric current: A. Copper phthalocyanine B. Cholesteryl benzene C. Benzene clathrate D. None of these E. All of these 4. Which of the following polymer backbone is good for biomedical applications? A. anhydride B. Urethane C. ester D. None of these E. All of these 5. Which of the following characteristics is associated with poly- (dimethylsiloxane)? A. Solubility in water B. insulator C. High glass transition temperature D. None of the above E. Stable above 700 degrees 6. Which type of material contains both glass transition temperature as well as melting point A. Amorphous B. microcrystalline C. crosslinked D. All of the Above E. None of the above 7. Aluminum is more stable to the atmosphere than iron because A. Aluminum is there at the bottom in the electrochemical series B. Less electrons than iron C. Oxidation of Aluminum provides stability D. None of these E. It does not fracture 8. Which BN has structured the same as graphite? A. Alpha-BN B. Beta-BN C. Gamma-BN D. Delta-BN E. Wurtzite-BN 9. Iron can be extracted from. A. Hematite B. Siderite C. None of these D. Magnetite E. All of these 10. In the Van der Waals Potential curve, what forces are represented by the nuclear-nuclear attraction component? A. The 10^12 term B. The 10^6 term C. The 10^18 term D. The 10^10 term E. The 10^25 term ANSWER KEY 11. Which of the following properties belongs to Ceramics: F. Soft and weak G. Hard and strong H. Hard and brittle I. Hard and tough J. None of these 12. Which of the following is superconductor in nature? F. Graphite G. C60 H. C60 with alkali cations I. C60 without cations J. None of these 13. Name the small molecule compound that generates stacks that conduct an electric current: F. Copper phthalocyanine G. Cholesteryl benzene H. Benzene clathrate I. None of these J. All of these 14. Which of the following polymer backbone is good for biomedical applications? F. anhydride G. Urethane H. ester I. None of these J. All of these 15. Which of the following characteristics is associated with poly- (dimethylsiloxane)? F. Solubility in water G. insulator H. High glass transition temperature I. None of the above J. Stable above 700 degrees 16. Which type of material contains both glass transition temperature as well as melting point F. Amorphous G. microcrystalline H. crosslinked I. All of the Above J. None of the above 17. Aluminum is more stable to the atmosphere than iron because F. Aluminum is there at the bottom in the electrochemical series G. Less electrons than iron H. Oxidation of Aluminum provides stability I. None of these J. It does not fracture 18. Which BN has structured the same as graphite? F. Alpha-BN G. Beta-BN H. Gamma-BN I. Delta-BN J. Wurtzite-BN 19. Iron can be extracted from. F. Hematite G. Siderite H. None of these I. Magnetite J. All of these 20. In the Van der Waals Potential curve, what forces are represented by the nuclear-nuclear attraction component? F. The 10^12 term G. The 10^6 term H. The 10^18 term I. The 10^10 term J. The 10^25 term