School of Physics and Astronomy
University of Birmingham
Year 3 Photonics Laboratory Report
December 2021
Measuring the refractive index and
polarisability of gases using a twinpolarisation Michelson interferometer
Lucy Brown
Supervisor: Dr Vincent Boyer
Lab partner: Anabel Dunn-Birch
Abstract
1
Introduction
Twin-polarisation Michelson interferometers produce interference of light by first splitting it into
two beams, which travel different path lengths, before allowing them to recombine. Preliminary
investigations are undertaken to explore the twin-polarisation Michelson interferometer; in one arm
of this set-up a reflector is attached to a piezoelectric crystal, which expands when a voltage is
applied. This is used to explain the theory behind the interferometer and how a path length
difference between its two arms allows the generation of a Lissajous figure. The main experiment
uses a similar twin-polarisation Michelson interferometer set-up, except the path length change is
instead caused by the introduction of a gas into an evacuated quartz cell in one arm, causing a
change in its refractive index. A Lissajous figure is generated and used to find the change in path
length; this allows determination of the refractive index and polarisability of the introduced gas. The
polarisability of a gas is the constant of proportionality between the dipole moment of its
constituent molecules or atom and the local electric field [solid state]
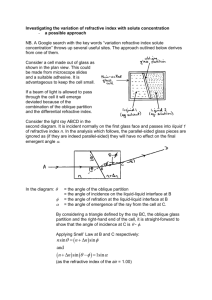
Preliminary investigations
Twin-polarisation Michelson interferometer with a piezoelectric crystal
The twin-polarisation Michelson interferometer utilises the two components of light to create
interference patterns on two photodiodes, allowing for the measurement of continuous
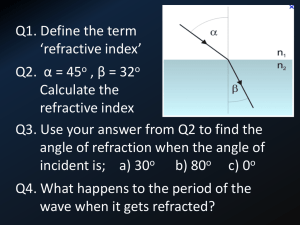
displacements of a reflector in one of the arms. Figure 1 shows a diagram of a twin-polarisation
Michelson interferometer used in this experiment. A 632.8 nm linearly polarised He-Ne laser is
incident on a beam splitter and split into two beams, one transmitted and one reflected. The
reflected beam travels to corner reflector R2, and is reflected back to the beam splitter, where part
of it is transmitted through to the polarising beam splitter. The other beam passes through the beam
splitter to corner reflector R1, is reflected back to the beam splitter, where some is reflected
towards the polarising beam splitter. The polarising beam splitter splits incoming light into its two
linearly polarised components; light that is polarised in the plane of incidence is transmitted to form
an interference pattern on photodiode D1, whereas light that is polarised perpendicular to the plane
of incidence is reflected to form an interference pattern on photodiode D2.
A property of the central beam splitter is that it causes a phase shift of π/2 of the reflected beam
with respect to the transmitted beam. Both beams undergo one reflection in the beam splitter, so
are shifted equivalently by λ/2. If the two optical paths differ by an integer number of wavelengths,
λ, then when the two beams combine again after the beam splitter, they are in phase and
constructive interference occurs. If one reflector is moved by λ/4, then since the light travels twice
this distance, the path difference is λ/2, and the beams are exactly out of phase when they combine
again after the beam splitter, so full destructive interference occurs. R2 is attached to a piezoelectric
crystal, which expands when a potential difference is applied, and the expansion is dependent on
this potential difference. Thus, by applying a potential difference across the piezoelectric crystal, the
optical path of the right-hand arm can be changed. This causes a change in path length difference
between the two beams and so the interference pattern moves. Changing the path length difference
by λ shifts the interference pattern such that each fringe is moved to the position of the next one, so
that each period of the pattern corresponds to a path length difference of λ. Since the beam travels
to the reflector and back again, this corresponds to a reflector displacement of λ/2.
As shown in figure 1, a function generator (FG) is connected to the piezoelectric crystal via a BNC
cable. This is used to apply a triangular voltage signal to the crystal, which has a voltage range of 0 <
V < +150V. As the voltage undergoes one cycle, the crystal expands and contracts, causing a
changing path length of the one arm and thus a changing path length difference of the two beams.
2
Figure 1: Diagram of twin-polarisation Michelson interferometer.
Photodiodes D1 and D1 are connected to the oscilloscope such that channels 1 and 2 are the optical
outputs from D1 and D2 respectively. Figure 2 shows the optical output from D1 and D2 against time
plotted on Python, with a visible phase difference of π/2.
Figure 2: Python plot of CH1 (optical output from D1) and CH2 (optical output from D1) voltage against time for twinpolarisation Michelson interferometer with a peak-to-peak voltage of 4.7 V across the piezoelectric crystal and a
frequency of 30.5 Hz.
Generating a Lissajous figure
Using the X-Y function on the oscilloscope, an elliptical Lissajous figure can be generated. A point will
rotate around this ellipse as the path length is changed. One period of rotation corresponds to a
path length difference of λ and therefore a change in the reflector’s displacement of λ/2. This can be
seen by varying the voltage across the crystal, causing a change in its size and thus the path length
difference. A point on the circle rotates differently for different translation directions: if the circle is
traced clockwise as the reflector is moving towards beam splitter, then the circle is traced
anticlockwise as the reflector is moving away from it. This is an advantage over the basic Michelson
interferometer, which only uses photodiode D1 and does not allow determination of the direction
R2 travels.
The point on the oscilloscope performs two harmonic oscillations in the two mutually perpendicular
directions: CH1 is traced along the x-axis and CH2 traced along the y-axis. The shape of the Lissajous
3
figure depends on the ratio of frequencies, phases and amplitudes of the two oscillations. In the
simplest case where their periods are equal, ellipses are generated which change eccentricity with
phase. When the phase difference is 0 or π the ellipse becomes a line segment. When the phase
difference is π/2 the ellipse becomes a circle. If the two oscillations have different periods, the phase
difference is constantly changing, meaning that the shape of the ellipse changes continuously.
Since the phase difference between the optical outputs from D1 (CH1) and D2 (CH2) is π/2, a circular
Lissajous figure was displayed on the oscilloscope and plotted using Python as shown in figure 3. The
Lissajous figure is a complete circle as the peak-to-peak voltage on the FG was set to a sufficiently
high value of 4.7 V. It was found that decreasing this voltage causes a smaller section of the circle to
be traced corresponding to less than one fringe. The frequency was set at 30.5 Hz so that the crystal
expands and contracts rapidly, causing the circle to be traced out rapidly. It was found that
decreasing the frequency causes the point to move slower around the circle, allowing it to be seen
tracing the circle on the oscilloscope.
Figure 3: Python plot of CH1 (optical output from D1) against CH2 (optical output from D2) for the twinpolarisation Michelson interferometer with a peak-to-peak voltage of 4.7 V across the piezoelectric crystal
and a frequency of 30.5 Hz.
Main experiment: measuring refractive index and
polarisability
The main experiment involves the use of a quartz cell, with optical windows at either end, placed in
one of the arms of the interferometer. A vacuum is created in the cell using a vacuum pump before
introducing a gas from a bladder, causing a change in refractive index and thus a change in path
length, instead of the use of a piezoelectric crystal. Figure 4 shows a diagram of the set-up used in
this experiment. The angle swept on the Lissajous figure can be used to find this change in path
length; this allows measurement of the refractive index and polarisability of the introduced gas.
FIGURE 4: DIAGRAM MAIN EXP (this will take up space on next page)
Theory
The angle swept on the Lissajous figure can be plotted against the number of data points taken. The
change in angle is given by:
4
𝛥𝜃 = |𝜃𝑚𝑎𝑥 − 𝜃𝑚𝑎𝑥 |
(1)
where 𝜃𝑚𝑎𝑥 and 𝜃𝑚𝑖𝑛 are the maximum and minimum values on this graph, respectively.
The change in angle divided by 2π gives the number of wavelengths the path length has changed by
during the introduction of the gas, so that multiplying by the wavelength of 632.8nm gives the path
length change in nm. Therefore, the change in optical path length is given by:
𝛥𝐿 = 𝛥𝜃 x
632.8
2𝜋
where ΔL = | 𝐿𝑔𝑎𝑠 − 𝐿𝑣𝑎𝑐 |
(2)
where 𝐿𝑔𝑎𝑠 and 𝐿𝑣𝑎𝑐 are the optical path lengths of the laser light travelling through the cell
containing a gas and vacuum respectively and are given by:
𝐿𝑔𝑎𝑠 = 2𝑑𝑛𝑔𝑎𝑠 and 𝐿𝑣𝑎𝑐 = 2𝑑𝑛𝑣𝑎𝑐 = 2𝑑,
(3)
where d is the length of the cell (d = 5cm from manufacturer’s information) and 𝑛𝑔𝑎𝑠 and 𝑛𝑣𝑎𝑐 =
1 are the refractive indices of the gas and vacuum respectively. The factor of 2 arises due to the light
travelling through the cell twice. This leads to the expression,
𝐿𝑣𝑎𝑐
𝐿𝑔𝑎𝑠
𝑛
= 𝑛𝑣𝑎𝑐 = 𝑛
𝑔𝑎𝑠
1
𝑔𝑎𝑠
⇒ 𝑛𝑔𝑎𝑠 =
𝐿𝑔𝑎𝑠
2𝑑
(4)
Adding and subtracting 𝐿𝑣𝑎𝑐 = 2𝑑 in the numerator gives:
𝑛𝑔𝑎𝑠 =
𝐿𝑔𝑎𝑠 − 𝐿𝑣𝑎𝑐 + 𝐿𝑣𝑎𝑐
2𝑑
=
ΔL+2d
2𝑑
ΔL
= 1 + 2𝑑 where ΔL = | 𝐿𝑔𝑎𝑠 − 𝐿𝑣𝑎𝑐 |.
(5)
The Clausius-Mossotti equation relates the dielectric constant of a material to the polarisability of its
constituent molecules or atoms and is given by:
ε𝑟 −1
ε𝑟 +2
=
4𝜋
3
𝑁𝛼
(6)
where ε𝑟 = ε/ε0 is the dielectric constant of the material (equal to n2 for non-magnetic materials),
N is the number of molecules per unit volume and 𝛼 is the polarisability.
Following this, the Lorentz-Lorenz equation relates the refractive index of a gas, 𝑛𝑔𝑎𝑠 , to the
polarisability of its constituent molecules or atoms and is given by:
𝑛𝑔𝑎𝑠 2 −1
𝑛𝑔𝑎𝑠 2 +2
=
4𝜋
3
𝑁𝛼 =
4𝜋 𝜌𝑁𝐴 𝛼
3
𝑀
(7)
where 𝑁𝐴 is Avogadro’s constant, 𝜌 is the mass density of the gas, M is its molar mass, and the
𝜌𝑁
relation 𝑁 = 𝑀𝐴 is used.
Under the assumption of an ideal gas, the ideal gas law can be used:
𝑃𝑉 = 𝑛𝑅𝑇
(8)
where P is the pressure of the gas, V is the volume taken up by the gas, T is the temperature of the
gas, R is the ideal gas constant and n is the number of moles of the gas. Since the volume taken up
the gas is given by its mass, m, divided by its density and the molar mass of the gas is given by M =
m/n, the density is given by:
𝑃𝑚
𝜌 = 𝑛𝑅𝑇 =
𝑃𝑀
.
𝑅𝑇
(9)
Thus, the polarisability of the gas can be written as:
𝛼=
3𝑅𝑇 𝑛𝑔𝑎𝑠 2 −1
4𝜋𝑁𝐴 𝑃 𝑛𝑔𝑎𝑠 2 +2
5
.
(10)
Method
A Measurement Computing Data Acquisition System (MCCDAQ) was used to rapidly take data from
the photodiodes, and the digital data was unpacked using Python. The analogue input range was set
to 1, allowing ± 10 V, and the Lissajous figure was centred at the origin. The np.arctan2() function
was used to find the angle between each data point and the positive x-axis. The np.unwrap()
function was used; the function continues to add 2π to the angle swept each time a circle is swept,
allowing the total angle swept in either direction to be found.
The method was as follows:
1. The quartz cell with length 5 cm was added to the right-hand arm of the twin-polarisation
Michelson interferometer.
2. Rubber tubing with a valve attached was connected to one opening on the cell. The valve
was placed at the end of the rubber tubing, so that it was as far from the cell as possible; this
was to minimise the effect of opening and closing it on the measurements, as the set-up is
very sensitive to vibrations and temperature.
3. The cell was evacuated using the vacuum pump connected to the other opening on the cell,
creating as close to vacuum as possible in the cell.
4. The code was started, and after 2 seconds, the valve was opened to introduce air from the
laboratory into the cell. The code was set to take data for 10 seconds.
5. The code generates the Lissajous figure and plots the angle swept against number of data
points and calculates the total angle swept using equation 1.
6. The change in path length was calculated using equation 2, and thus the refractive index of
air in the cell using equation 5.
7. Temperature was recorded from a thermometer in the lab as 20 ℃. Atmospheric pressure of
101325 Pa was used for the pressure in the cell. Using these values, the polarisability of air
molecules in the cell was calculated using equation 10.
8. Steps 2-4 were repeated 15 times. The mean values of refractive index and polarisability of
the air in the cell were calculated.
9. Steps 2-5 were repeated for the investigation of helium, argon and nitrogen, using a bladder
containing the gas, which was attached to the cell with a valve.
Results and analysis
Figure 5 shows a graph of total angle swept against the number of data points taken for each of the
gases measured. The graphs for air, argon and nitrogen look similar, all having a very small positive
gradient, followed by a large positive gradient which then plateaus. The large increase in angle
swept arises from the Lissajous figure being swept when introducing the gas into the cell, which
increases the pressure and refractive index in the cell. This causes the angle swept to be positive.
However, the graph for helium differs in that there is a small sharp upwards spike followed by a
larger sharp downwards spike, before the larger increase. This arises from the opening of the valve.
The downwards spike corresponds to the Lissajous figure being swept in the reverse direction,
causing a negative change in angle, indicating the pressure and refractive index in the cell is
decreasing. The plateau for air, argon and nitrogen were smooth for most of the repeats, whereas
for helium it was not.
It was found that the spikes in figure 5a for helium still appeared when only opening the valve from
the bladder to 45°, so that helium is introduced into the cell slower. Helium has a molar mass of
4.003 g mol-1, which is significantly lower than the molar mass of air, 28.966 g mol-1[molar masses
ref]. These spikes did not appear for argon and nitrogen, which have greater molar masses than air,
6
39.948 g mol-1 and 28.013 g mol-1 respectively. This suggests that mechanical pressure differences
that occur when helium is introduced into the cell are responsible, which only occur when the molar
mass of the introduced gas is lighter than that of air.
The graphs in figure 5 should theoretically start at zero; due to the fluctuations, it does not, but data
points are taken so quickly that the first points should be very close to zero and indeed they were.
The code takes the minimum value of angle, which would be the bottom of the downwards spike for
helium, hence the minimum angle was set to zero instead.
a)
b)
Total angle swept as data is taken for air
c)
Total angle swept as data is taken for helium
d)
Total angle swept as data is taken for argon
Total angle swept as data is taken for nitrogen
Figure 5: Graph of total angle swept against the number of data points taken for a) air, b) helium,
c) argon and d) nitrogen. The number of data points taken was 40000 at a rate of 4000 s-1, giving a
total time of 10 s.
Under the simplification that air is composed of 78% nitrogen and 22% oxygen, we can use the
measured refractive indices of air and nitrogen, 𝑛𝑎𝑖𝑟 and 𝑛𝑁 respectively, to predict the refractive
index of oxygen, 𝑛𝑂 . Consider a cell containing the mixture of these two gases compared to a cell
with 78% nitrogen separated at one end and 22% oxygen separated at the other end. Since the light
is traveling through the same number of molecules of each gas in both cases, it is predicted that the
refractive index will be the same in these cases. The optical path length of light travelling through
the cell filled with air is:
𝐿𝑎𝑖𝑟 = 2𝑑𝑛𝑎𝑖𝑟 = 2𝑑 (0.78𝑛𝑁 + 0.22𝑛𝑂 ).
From this, it follows that the refractive index of oxygen can be calculated by:
7
(11)
𝑛𝑂 =
𝑛𝑎𝑖𝑟
0.22
− 𝑛𝑁
0.78
.
0.22
(12)
Table 1 shows the mean values of refractive index and polarisability along with their errors for air,
helium, argon and nitrogen, as measured in this experiment. The predicted refractive index of
oxygen, along with its error, is also included. The experimental refractive indices measured are much
closer to the literature values than the values of polarisability are. The experimental mean and
literature value for the refractive index of helium agree within its experimental error; for the other
gases, this is not the case, but they are very close. This is surprising as the graph of angle swept for
helium in figure 5 was much less smooth and had unexpected spikes compared to the graphs for the
other gases. The refractive index for helium was found to be an order of magnitude lower than that
of the other gases; this follows from its graph in figure 5 where the angle swept is an order of
magnitude lower than that of the other gases. The errors on the polarisability were large, but still
the experimental mean did not agree with the literature value within experimental error. The
experimental values of polarisability differed less than the literature values do. The accuracy of the
experiment in measuring the polarisability was therefore lower than it was for measuring the
refractive index.
Table 1: Experimental values of refractive index and polarisability of various gases, compared to
literature values for 20 ℃ and 632.8 nm.
Gas
Refractive index
Polarisability (10-30 m3)
Mean
Error
Literature value Mean
Error Literature value
Air
1.000249 0.000008
1.000272 [A]
1.546
0.514
21.180 [B]
Helium
1.000032 0.000009
1.000035 [C]
1.996
0.609
0.208 [D]
Argon
1.000239 0.000009
1.000263 [E]
1.448
0.579
6.876 [F]
Nitrogen 1.000256 0.000007
1.000280 [E]
1.594
0.450
1.100 [D]
Oxygen* 1.000221 0.000011
1.000251 [G]
*The refractive index of oxygen was predicted from that of air and nitrogen and was not directly
measured in this experiment.
One source of error in the data was the background noise from the photodiodes, affecting the
change in angle from the graphs. Data was taken for 10 s increments with 10 repeats of only the
background noise (no refilling or evacuating the cell). The absolute difference between the
maximum and minimum values divided by two gave the error; the mean of these were used as the
error on the maximum or minimum values, σ(θ) = 1.662464. The error on the change in angle was
calculated using:
𝛥θ = √σ(θ)2 + σ(θ)2 = √2 σ(θ).
The error on the change in path length was calculated by:
σ(𝛥𝐿) =
632.8
2𝜋
σ(𝛥θ).
It was assumed that there was a negligible error on d, as this was given by the manufacturer. Thus,
the error on 𝑛𝑔𝑎𝑠 was calculated by:
σ(𝑛𝑔𝑎𝑠 ) = √(1 +
𝛥𝐿 2
1 2
𝛥𝐿 2
) σ(𝑛𝑣𝑎𝑐 )2 + ( ) σ(𝛥𝐿)2 + ( 2 ) 4𝑑2 σ(𝑛𝑣𝑎𝑐 )2
2𝑑
2𝑑
4𝑑
The error on the refractive index of the vacuum arises since the vacuum pump would not create a
perfect vacuum in the cell and tubing, so that its refractive index would be slightly greater than 1.
The estimate of σ(𝑛𝑣𝑎𝑐 ) was 10-7, three orders of magnitude lower than most of the refractive
8
indices minus one, except helium, as found in the experiment. However, this error may have been
underestimated as if there was more residual gas left in the cell than expected, it could have been
closer to 10-5, the order of magnitude that the refractive indices, except helium, differed by.
It was assumed that there was a negligible error on P as only the atmospheric pressure was used.
The error on temperature was error was taken as 0.5 ℃, from half the resolution of the
thermometer. The error on temperature in Kelvin was then 0.5 K, since 1 ℃ = 1 K. Thus, the error on
𝛼 was calculated by:
2
2
𝑛 2−1
𝑛𝑔𝑎𝑠 2 − 1
3𝑅
2+2−
√( 𝑔𝑎𝑠
σ(𝛼) =
σ(𝑇))
+
(2𝑛
𝑇
{𝑛
} σ(𝑛𝑔𝑎𝑠 )) .
𝑔𝑎𝑠
𝑔𝑎𝑠
4𝜋𝑁𝐴
𝑛𝑔𝑎𝑠 2 + 2
(𝑛𝑔𝑎𝑠 2 + 2)2
However, it was not accounted for was that the bladder was initially under tension at a pressure
higher than atmospheric. Then, as repeats were taken, the pressure gradually decreased, and after
many repeats there was little gas left in the bladder. Atmospheric pressure was used for all repeats
and so this error was not accounted for, but it would have affected the first few measurements in
particular. This could have contributed to a low accuracy in measuring the polarisability and thus the
difference between experimental and literature values in table 1.
In addition, it was noticed that the temperature read from the thermometer was the same each day
measurements were taken; as this is unlikely to be true, the thermometer may not have been
accurate, and so a larger error was needed. This also indicates that the experimental and literature
values may have been compared at different temperatures and since the refractive index and
polarisability depend on the temperature, this could explain the difference.
An issue when completing the experiment was the effectiveness of the seals created, particularly
between the cell and rubber tubing. To test for leaks, the code was run for a longer time of 150 s
without opening the valve that introduces the gas. A change in angle swept over this time indicated
that air was refilling the cell. A graph testing this is shown in figure 6 for the set-up for measuring air.
Over the 150 s, the cell begins to refill with air as there is a positive gradient, but as it does not
plateau, it has not completely refilled. For every second delay in between evacuating and refilling
the cell, the angle is sweeping 0.8 radians; for this reason, data was taken as quickly as possible. As
the code runs for 10 s, the angle swept due to leaks is estimated to be 10 radians. This would add to
the angle swept from the introduction of the gas, causing inaccuracies in measuring the path length
change and thus refractive index and polarisability. As air from the room fills the cell, it mixes with
the gas introduced, causing a change in the refractive and polarisability of the gas in the cell, but this
would be very small.
9
Total angle swept against number of data points
Figure 6: Graph of total angle swept as data is taken with valves shut, testing for leaks in the setup used for investigating air. The number of data points taken was 60000 at a rate of 400 s-1, giving
a total time of 150 s.
Conclusion
Main sources inaccuracies: Error on the refractive index of the vacuum and the pressure on the
bladder may have been much greater than expected. Leaking may have also been a source of
inaccuracy.
Further investigation measure for gases lighter than air to see if spikes
References
[1]
[A] https://emtoolbox.nist.gov/Wavelength/Documentation.asp
[B] https://aapt.scitation.org/doi/10.1119/1.3533354
[C] https://refractiveindex.info/?shelf=main&book=He&page=Mansfield
[D] https://cccbdb.nist.gov/pollistx.asp#1977Mil/Bed:1
[E] https://pubs.acs.org/doi/pdf/10.1021/je980133o
[F] https://journals.aps.org/prl/abstract/10.1103/PhysRevLett.120.123203
[G] Refractive index of O2 (Oxygen) - Zhang
10