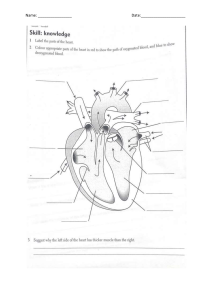
Chapter 35 – Care of the Patient with Acute Coronary Syndromes acute coronary syndrome (ACS) Disorder including unstable angina and myocardial infarction; results from obstruction of the coronary artery by ruptured atherosclerotic plaque and leads to platelet aggregation, thrombus formation, and vasoconstriction. angina pectoris Chest pain caused by a temporary imbalance between the coronary arteries’ ability to supply oxygen and the cardiac muscle’s demand for oxygen. atypical angina Angina with vague presentation such as indigestion, pain between the shoulders, an aching jaw, or choking sensation that occurs with exertion. cardiac rehabilitation The process of actively assisting the patient with coronary disease to achieve and maintain a productive life while remaining within the limits of the heart’s ability to respond to increases in activity and stress. cardiogenic shock Post–myocardial infarction heart failure in which necrosis of more than 40% of the left ventricle has occurred; also called Class IV heart failure. chronic stable angina (CSA) Type of angina characterized by chest discomfort that occurs with moderate-to-prolonged exertion and in a pattern that is familiar to the patient. coronary artery bypass graft (CABG) Surgical procedure in which occluded arteries are bypassed with the patient’s own venous or arterial blood vessels or synthetic grafts. coronary artery disease (CAD) Disease affecting the arteries that provide blood, oxygen, and nutrients to the myocardium; also known as coronary heart disease or simply heart disease. infarction Necrosis, or cell death. intra-aortic balloon pump (IABP) Specialized catheter and balloon inserted into aorta that inflates and deflates with the cardiac cycle in order to decrease afterload and increase coronary perfusion. ischemia Blockage of blood flow through a blood vessel, resulting in a lack of oxygen. metabolic syndrome Collection of related health problems with insulin resistance as a main feature; increases the risk for cardiovascular disease. myocardial infarction (MI) Injury and necrosis of myocardial tissue that occurs when the tissue is abruptly and severely deprived of oxygen. new-onset angina Cardiac chest pain that occurs for the first time. non–ST-elevation myocardial infarction (NSTEMI) Myocardial infarction in which the patient typically has ST- and T-wave changes on a 12-lead ECG; this indicates myocardial ischemia. percutaneous coronary intervention (PCI) Nonsurgical method of improving arterial flow by opening the vessel lumen. A balloon is inserted in the coronary artery and inflated to open blood vessels; procedure may include insertion of a coronary stent. pulmonary artery occlusion pressure (PAOP) Measurement of pressure in the left atrium using a balloon-tipped catheter introduced into the pulmonary artery. ST-elevation myocardial infarction (STEMI) Myocardial infarction in which the patient typically has ST elevation in two contiguous leads on a 12-lead ECG; this indicates myocardial infarction (necrosis). vasospastic angina Angina caused by coronary artery vasospasm that often occurs at rest or during the early morning hours; also called variant or Prinzmetal angina. ventricular remodeling After a myocardial infarction, permanent changes in the size and shape of the left ventricle due to scar tissue; such remodeling can decrease left ventricular function and cause heart failure. Coronary Artery Disease (CAD) - Chronic stable angina, acute coronary syndromes - Ischemia - Infarction Coronary artery disease (CAD) is a broad term that includes chronic stable angina and acute coronary syndrome (ACS). It affects the arteries that provide blood, oxygen, and nutrients to the myocardium. When blood flow through the coronary arteries is partially or completely blocked, ischemia and infarction of the myocardium may result. Ischemia occurs when insufficient oxygen is supplied to meet the requirements of the myocardium. Infarction (necrosis, or cell death) occurs when severe ischemia is prolonged and decreased perfusion causes irreversible damage to tissue. CAD, also called coronary heart disease (CHD) or simply heart disease, is the single largest killer of American men and women in all ethnic groups. When the arteries that supply the myocardium are diseased, the heart cannot pump blood effectively to adequately perfuse vital organs and peripheral tissues. The organs and tissues need oxygen in arterial blood for survival. When perfusion is impaired, the patient can have life-threatening signs and symptoms and possibly death. Chronic Stable Angina (CSA) Pectoris Angina pectoris is chest pain caused by a temporary imbalance between the coronary arteries’ ability to supply oxygen and the cardiac muscle’s demand for oxygen. Ischemia (lack of oxygen) that occurs with angina is limited in duration and does not cause permanent damage of myocardial tissue. Angina may be of two main types: stable angina and unstable angina. Chronic stable angina (CSA) is chest discomfort that occurs with moderate-to-prolonged exertion in a pattern that is familiar to the patient. The frequency, duration, and intensity of symptoms remain the same over several months. CSA results in only slight limitation of activity and is usually associated with a fixed atherosclerotic plaque. It is usually relieved by nitroglycerin (NTG) or rest and often is managed with drug therapy. Rarely does CSA require aggressive treatment. Perfusion Concept Exemplar: Acute Coronary Syndrome Pathophysiology Review The term acute coronary syndrome (ACS) is used to describe patients who have either unstable angina or an acute myocardial infarction (MI). In ACS, it is believed that the atherosclerotic plaque in the coronary artery ruptures, resulting in platelet aggregation (“clumping”), thrombus (clot) formation, and vasoconstriction. The amount of disruption of the atherosclerotic plaque determines the degree of coronary artery obstruction (blockage) and the specific disease process. Once the artery reaches 50% occlusion, blood flow is impaired, creating myocardial ischemia when myocardial demand is increased. Unstable angina (UA) is chest pain or discomfort that occurs at rest or with exertion and causes severe activity limitation. An increase in the number of attacks and in the intensity of the pressure indicates UA. The pressure may last longer than 15 minutes or may be poorly relieved by rest or nitroglycerin. Patients with unstable angina may present with ST changes on a 12-lead ECG but do not have changes in troponin levels. Ischemia is present but is not severe enough to cause detectable myocardial damage or cell death. As the assays for troponins become more sensitive, the diagnosis of UA is decreasing. UA includes: - New-onset angina – when a patient has their first angina symptoms, usually after exertion or other increased demands on the heart. - Vasospastic angina (variant or Prinzmetal angina) - chest pain or discomfort resulting from coronary artery spasm and typically occurs after rest - Pre-infarction angina - chest pain that occurs in the days or weeks before a myocardial infarction. The most serious acute coronary syndrome is myocardial infarction (MI), often referred to as acute MI or AMI. Undiagnosed or untreated angina can lead to this very serious health problem. Myocardial infarction (MI) occurs when myocardial tissue is abruptly and severely deprived of oxygen. When blood flow is quickly reduced by 80% to 90%, ischemia develops. Ischemia can lead to injury and necrosis of myocardial tissue if blood flow is not restored. Two types of MI: non–ST-segment elevation myocardial infarction (NSTEMI) and ST-elevation myocardial infarction (STEMI). NSTEMI (non–ST-segment elevation myocardial infarction) - Typically have ST segment and T-wave changes on a 12-lead ECG (ST depression and T-wave3 inversion), this indicates myocardial ischemia - Troponin may be normal, but it elevates over the next 3 to 12 hours. o This combo indicates myocardial cell death or necrosis o - Asses clinical presentation and history of patient – patients without typical symptoms (chest discomfort, shortness of breath, nausea) of ACS may have another condition (ex. Sepsis) Causes of NSTEMI o Coronary vasospasm o Spontaneous dissection o Sluggish blood flow due to narrowing of the coronary artery. STEMI (ST-elevation myocardial infarction) - ST elevation in two contiguous leads on a 12-lead ECG (Indicates MI/necrosis) - Attributable to rupture of the fibrous atherosclerotic plaque leading to platelet aggregation and thrombus formation at the site of rupture - The thrombus causes an abrupt 100% occlusion to the coronary artery; this is a medical emergency and requires immediate revascularization of the blocked coronary artery. Often MIs begin with infarction of the subendocardial (inner ventricular walls) layer of cardiac muscle, which has the greatest oxygen demand and the poorest oxygen supply. Zone of necrosis - Initial area of infarction Zone of injury - tissue that is injured but not necrotic Zone of ischemia—tissue that is oxygen deprived - Infarction is a dynamic process that evolves over several hours. Hypoxemia from ischemia may lead to local vasodilation of blood vessels and acidosis. Potassium, calcium, and magnesium imbalances, as well as acidosis at the cellular level, may cause changes in normal conduction and contractile functions. Catecholamines (epinephrine and norepinephrine) released in response to hypoxia and pain may increase the heart’s rate, contractility, and afterload. - These factors increase oxygen requirements in tissue that is already oxygen deprived. - This may lead to life-threatening ventricular dysrhythmias. - The area of infarction may extend into the zones of injury and ischemia. - The zone of infarction depends on three factors o Collateral circulation o Anaerobic metabolism o Workload demands on the myocardium. - Obvious physical changes occur 6 hours after the infarction - infarcted region appears blue/ swollen – need for intervention within first 4-6 hours of symptom onset After 48 hours – are turns gray with yellow streaks as neutrophils remove the necrotic cells 8-10 days after, granulation tissue forms at the edges of the necrotic tissue 2-3 months – necrotic area develops into a shrunken, thin, firm scar. Scar tissue causes ventricular remodeling (permanently changes the size and shape of the left ventricle) o May decrease left ventricular function (lead to hear failure, morbidity, and mortality) o Scar tissue does not contract or conduct electrically – often cause of chronic ventricular dysrhythmias The patient’s response to an MI also depends on which coronary artery or arteries were obstructed and which part of the ventricle wall was damaged: anterior, septal, lateral, inferior, or posterior. - - - Obstruction of the left anterior descending (LAD) artery causes anterior or septal MIs because it perfuses the anterior wall and most of the septum of the left ventricle. Patients with anterior wall MIs (AWMIs) have the highest mortality rate because they are most likely to have left ventricular failure and dysrhythmias from damage to the left ventricle. The circumflex artery supplies the lateral wall of the left ventricle and possibly portions of the posterior wall or the sinoatrial (SA) and atrioventricular (AV) nodes. Patients with obstruction of the circumflex artery may experience a posterior wall MI (PWMI) or a lateral wall MI (LWMI) and sinus dysrhythmias. In most people, the right coronary artery (RCA) supplies most of the SA and AV nodes, as well as the right ventricle and inferior or diaphragmatic portion of the left ventricle. Patients with obstruction of the RCA often have inferior wall MIs (IWMIs). About half of all IWMIs are associated with an occlusion of the RCA, causing significant damage to the right ventricle. Thus it is important to obtain a “right-sided” ECG to assess for right ventricular involvement Etiology and Genetic Risk Atherosclerosis (A thickening or hardening of the arterial wall, often associated with aging) is the primary factor in the development of CAD. Nonmodifiable Risk Factors - Age - Gender - Family History - Ethnic Background Modifiable Risk Factors - Smoking - Obesity - Stress - Elevated Cholesterol - Hypertension - Diabetes Metabolic syndrome (insulin resistance syndrome or syndrome X) is a risk factor for cardiovascular (CV) disease. Patients who have three of the factors in the below table are diagnosed with metabolic syndrome. This health problem increases the risk for developing diabetes and CAD. The presence of central obesity, high blood pressure, and hyperglycemia when diagnosed with metabolic syndrome presents the highest risk for development of cardiovascular disease. Females have a higher prevalence of metabolic syndrome, and overall prevalence increases with age. Health Promotion and Maintenance ◦ AEDs (automatic external defibrillators) available in public places and in homes ◦ Controlling or altering modifiable risk factors is critical ◦ Action ◦ ◦ Create a patient teaching handout that incorporates a list of modifiable and nonmodifiable risk factors for coronary artery disease (CAD). Include pertinent teaching information about how patients could modify behaviors to address modifiable risk factors. Assessment: Recognize Cues History: If symptoms of CAD are present at the time of the interview, delay collecting data until interventions are started to relieve symptoms. Physical Assessment/Signs and Symptoms: - Rapid assessment is crucial - Differentiate among the types of chest pain and identify the source - Assess blood pressure, heart rate, distal peripheral pulses, skin temperature, respiratory rate, breath sounds, presence of jugular venous distention and peripheral edema - Auscultate for an S 3 gallop, which often indicates heart failure Psychosocial Assessment: - Assess the patient’s current coping mechanisms - Denial, anger, depression, fear, and anxiety are common Laboratory Assessment: - There is no single test to diagnose MI - Most common laboratory tests include troponins T and I - Troponin is specific for MI and cardiac necrosis - Troponins T and I rise quickly Imaging Assessment: - A chest x-ray may be performed to help rule out aortic dissection, which may mimic an MI. - Thallium scans use radioisotope imaging to assess for ischemia or necrotic muscle tissue related to angina or MI. Areas of decreased or absent perfusion, referred to as cold spots, identify ischemia or infarction. Thallium may be used with the exercise tolerance test.Dipyridamole thallium scanning (DTS) may also be used. - Contrast-enhanced cardiovascular magnetic resonance (CMR) imaging may also be done as a noninvasive approach to detect CAD - Echocardiography may be used to visualize the structures of the heart - Use of 64-slice computed tomography coronary angiography (CTCA) has been found to be helpful in diagnosing CAD. The high-speed CT scanner is a highly reliable, noninvasive way to evaluate calcified plaque. This plaque is then quantified into the calcium score. Those with a calcium score (also called the Agatston score) higher than 400 have a higher risk of developing myocardial infarction and death within the next 2 to 5 years Other Diagnostic Assessment: - Twelve-lead ECGs examine the heart from varying perspectives - Identifying the lead(s) in which ECG changes are occurring can identify both the occurrence and the location of ischemia (angina) or necrosis (infarction). - “Right-sided” or 18-lead ECG can determine whether ischemia or infarction have occurred in the right ventricle - The ECG should be obtained within 10 minutes of patient presentation with chest discomfort - 12-lead ECGs obtained during an angina episode reveal ST depression, T-wave inversion, or both. - - Vasospastic angina, caused by coronary vasospasm (vessel spasm), usually causes elevation of the ST segment during angina attacks. These ST and T-wave changes usually subside when the ischemia is resolved and pain is relieved. However, the T wave may remain flat or inverted for a period of time. When infarction occurs, one of two ECG changes is usually observed: ST elevation MI (STEMI), or non–ST-elevation MI (NSTEMI). An abnormal Q wave (wider than 0.04 second or more than one-third the height of the QRS complex) may develop, depending on the amount of myocardium that has necrosed. After the acute stages of an unstable angina episode, the health care provider often requests an exercise tolerance test (stress test) on a treadmill to assess for ECG changes consistent with ischemia, evaluate medical therapy, and identify those who might benefit from invasive therapy. Pharmacologic stress-testing agents such as dobutamine may be used instead of the treadmill Cardiac catheterization may be performed to determine the extent and exact location of coronary artery obstructions. Identifies patients who might benefit from percutaneous coronary intervention (PCI) or from coronary artery bypass graft (CABG) Analysis: Analyze Cues and Prioritize Hypotheses: The priority collaborative problems for most patients with acute coronary syndrome (ACS) include: 1. Acute pain due to an imbalance between myocardial oxygen supply and demand 2. Decreased myocardial tissue perfusion due to interruption of arterial blood flow 3. Potential for dysrhythmias due to ischemia and ventricular irritability 4. Potential for heart failure due to left ventricular dysfunction Planning and Implementation: Generate Solutions and Take Action Managing Acute Pain - Decrease pain, decrease myocardial oxygen demand, and increase perfusion (myocardial oxygen supply). - The patient may take nitroglycerin to relieve episodic anginal pain. - Nitroglycerin is contraindicated with the use of phosphodiesterase inhibitors (used for erectile dysfunction or pulmonary arterial hypertension) because it can cause profound hypotension - Nitroglycerin (NTG), a nitrate often referred to as “nitro,” increases collateral blood flow, redistributes blood flow toward the subendocardium, and dilates the coronary arteries - During administration of long-term oral and topical nitrates, an 8- to 12-hour nitrate-free period should be maintained to prevent tolerance Increasing Myocardial Tissue Perfusion - The primary outcome is that the patient will have increased myocardial perfusion as evidenced by adequate cardiac output, normal sinus rhythm, and vital signs within normal limits - Aspirin inhibits both platelet aggregation and vasoconstriction, thereby decreasing the likelihood of thrombosis - P2Y 12 platelet inhibitors such as clopidogrel or ticagrelor, may be given with an initial loading dose followed by a daily dose for up to 12 months after diagnosis. These oral agents work to prevent platelets from aggregating (clumping) together to form clots - Glycoprotein (GP) IIb/IIIa inhibitors such as abciximab, eptifibatide, or tirofiban may be administered IV to prevent fibrinogen from aaching to activated platelets at the site of a thrombus. These medications are used in unstable angina and NSTEMI. They are also given before and during percutaneous coronary intervention (PCI) to maintain patency of an artery with a large clot and are given with fibrinolytic agents after STEMI - - - - - Another antiplatelet, a protease-activated receptor inhibitor (PAR-1), vorapaxar, is shown to decrease the risk of recurrent MI when added to the regimen of aspirin and clopidogrel Anticoagulation therapy may also be used to prevent clot formation Once-a-day beta-adrenergic blocking agents (e.g., metoprolol XL, carvedilol CR), sometimes just called beta blockers (BBs), decrease the size of the infarct, the occurrence of ventricular dysrhythmias, and mortality rates in patients with MI. Beta blockers slow the heart rate and decrease the force of cardiac contraction. Thus, these agents prolong the period of diastole and increase myocardial perfusion while reducing the force of myocardial contraction. With beta blockade, the heart can perform more work without ischemia. Health care providers frequently prescribe angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) within 24 hours of ACS to prevent ventricular remodeling and the development of heart failure For patients with angina, the health care provider may prescribe calcium channel blockers (CCBs) to promote vasodilation and myocardial perfusion. These drugs are indicated for patients with vasospastic angina or for those who are hypertensive and continue to have angina despite therapy with beta blockers (unstable angina). They are not indicated after an acute MI unless beta blockade is contraindicated Calcium channel blockers are also used for chronic stable angina (CSA). When they are not successful in managing CSA, ranolazine may be added to the drug regimen. This drug has antiangina and anti-ischemic properties and is often effective in relieving the pain associated with CSA Statin therapy reduces the risk of developing recurrent MI, mortality, and stroke Reperfusion Therapy - One of two reperfusion strategies are used to open a blocked artery in a patient experiencing acute MI: thrombolytic therapy or percutaneous coronary intervention (PCI). PCI is the treatment of choice for most patients with STEMI - Fibrinolytic therapy (also called thrombolytic therapy) dissolves thrombi in the coronary arteries and restores myocardial blood flow. - The goal is to administer fibrinolytic therapy within 30 minutes of arrival to the hospital - It is not indicated for the NSTEMI patient population - Monitor the patient for indications that the clot has been lysed (dissolved) and the artery reperfused. These indications include: o Abrupt cessation of pain or discomfort o Sudden onset of ventricular dysrhythmias o Resolution of ST-segment depression/elevation or T-wave inversion o A peak at 12 hours of markers of myocardial damage Percutaneous Coronary Intervention - - - - An invasive but nonsurgical technique that is the treatment of choice to reopen the clotted coronary artery and restore perfusion. The goal is to perform PCI within 90 minutes of an acute STEMI diagnosis Performed in the cardiac catheterization laboratory and combines clot retrieval, coronary angioplasty, and stent placement. Under fluoroscopic guidance, the cardiologist performs initial coronary angiography, inserting an arterial sheath and advancing a catheter in a retrograde manner through the aorta In the STEMI patient, if a clot is seen, a clot retrieval device is inserted over the guidewire, and the clot is removed Once the clot is removed in the STEMI patient or area of narrowing is identified in the NSTEMI patient, a balloon-tipped catheter is introduced through a guidewire to the coronary artery occlusion. The physician activates a compressor that inflates the balloon (angioplasty) to force the plaque against the vessel wall, thus dilating the wall, and reduces or eliminates the occluding clot. Balloon inflation may be repeated until angiography indicates a decrease in the stenosis (narrowing) to less than 50% of the vessel’s diameter The balloon catheter is then withdrawn, and a balloon catheter with stent is introduced. Once the stent and balloon are in position, the stent is deployed by the balloon inflation. The balloon is deflated and the stent stays in place, acting as scaffolding to hold the diseased artery open. Stents are expandable metal mesh devices that are used to maintain the patent lumen created by angioplasty or atherectomy - PCI initially reopens the vessel in most patients. However, within the first 24 hours, a small percentage of patients have restenosis. At 6 months, a larger number have one or more blockages. Without stent placement, the artery often reoccludes because of its normal elasticity and memory. Identifying and Managing Dysrhythmias - Dysrhythmias are the leading cause of prehospital death in most patients with ACS. - When a dysrhythmia develops: o Identify the dysrhythmia o Assess hemodynamic status o Evaluate for discomfort. - Dysrhythmias are treated when they cause hemodynamic compromise, increase myocardial oxygen requirements, or predispose the patient to lethal ventricular dysrhythmias - Typical dysrhythmias for the patient with an inferior ACS are bradycardias and second-degree atrioventricular (AV) blocks resulting from ischemia of the AV node. - If the patient becomes hemodynamically unstable, a temporary pacemaker may be necessary. - The patient with an anterior ACS is likely to exhibit premature ventricular contractions (PVCs) caused by ventricular irritability. Third-degree or bundle branch block is a serious complication in this patient because it indicates that a large portion of the left ventricle is involved. The health care provider may insert a pacemaker. Monitoring for and Managing Heart Failure - Decreased cardiac output due to heart failure is a relatively common complication after an MI resulting from left ventricular dysfunction, rupture of the intraventricular septum, papillary muscle rupture with valvular dysfunction, or right ventricular infarction. - The most severe form of acute heart12 failure, cardiogenic shock, causes most in-hospital deaths after an ACS Managing Left Ventricular Failure - The amount of blood that the heart can eject is reduced - When volume and pressure are markedly increased in the pulmonary vasculature, pulmonary complications such as, pulmonary edema, can develop - Hemodynamic monitoring is a term that refers to a variety of monitoring techniques designed to provide quantitative information about vascular capacity, blood volume, pump effectiveness, and tissue perfusion. The type of monitoring can vary from noninvasive to highly invasive. Finger cuff hemodynamic monitoring systems provide noninvasive continuous monitoring of stroke volume, cardiac output, and blood pressure. - Invasive hemodynamic monitoring directly measures pressures in the heart and great vessels. These procedures are usually performed for more seriously ill patients and can provide more accurate measurements of blood pressure, heart function, and volume status - Invasive hemodynamic monitoring does involve significant risks; informed consent is therefore required. The components of this pressure-monitoring system are a catheter with an infusion system, a transducer, and a monitor. The catheter receives the pressure waves (mechanical energy) from the heart or the great vessels. The transducer converts the mechanical energy into electrical energy, which is displayed as waveforms or numbers on the monitor. Patency of the catheter is maintained with a slow continuous flush of normal saline, usually infused at 3 to 4 mL/hr under pressure to prevent the backup of blood and occlusion of the catheter. - - - Because this system is designed to measure pressure, it is important to account for atmospheric pressure (calibration) and hydrostatic pressure associated with the level of the transducer. \ Identify the phlebostatic axis, a physical reference point on the chest, and level the transducer to this point Right atrial pressure is measured by a pressure sensor on the catheter inside the right atrium (RA). Normal RA pressure ranges from 0 to 8 mm Hg. Increased RA pressures may occur with right ventricular failure, whereas low RA pressures usually indicate hypovolemia. Pulmonary artery pressure (PAP) is also assessed and is constantly visible on most hemodynamic monitors. Normal PAP ranges from 15 to 30 mm Hg systolic to 3 to 12 mm Hg diastolic. When the balloon at the catheter tip is inflated, the catheter advances and wedges in a branch of the pulmonary artery. The tip of the catheter can sense pressures transmitted from the left atrium, which reflect left ventricular end-diastolic pressure (LVEDP). The pressure measured during balloon inflation is called the pulmonary artery occlusion pressure (PAOP), also referred to as a wedge pressure because the balloon is wedged within the small vessel. Normal PAOP ranges from 5 to 12 mm Hg. Elevated PAOP measurements may indicate left ventricular failure, hypervolemia, mitral regurgitation, or intracardiac shunting. A decreased PAOP is seen with hypovolemia or afterload reduction. Classification of post–myocardial infarction heart failure - The classic Killip system identifies four classes based on prognosis o Patients with class I heart failure often respond well to reduction in preload with IV nitrates and diuretics. Monitor the urine output hourly, check vital signs hourly, continue to assess for signs of heart failure, and review the serum potassium level. o Patients with class II and class III heart failure may require diuresis and more aggressive medical intervention, such as afterload reduction and/or enhancement of contractility. IV nitroprusside or nitroglycerin may be used to decrease both preload and afterload. These drugs are given as continuous infusions in specialized units where hemodynamic monitoring can occur. Intra-arterial BP monitoring is preferred for nitroprusside administration o Patients in classes II and III are usually started on once-a-day beta blockers. Dosing is titrated, depending on goal achievement and drug tolerance. Other drugs, including ACEIs and ARBs, are commonly prescribed to inhibit ventricular remodeling o Class IV heart failure is cardiogenic shock. In cardiogenic shock, necrosis of more than 40% of the left ventricle occurs. Most patients have a stuttering pattern of chest pain, resulting in extension of the ACS. Other interventions for left-sided heart failure - - - - - When patients do not respond to drug therapy with improved tissue perfusion, decreased workload of the heart, and increased cardiac contractility, mechanical circulatory support, such as an intra-aortic balloon pump (IABP) may be inserted. The IABP is a temporary, invasive, percutaneous intervention that is used to improve myocardial perfusion during an acute MI, reduce preload and afterload, and facilitate left ventricular ejection. Inflation of the IABP during diastole augments the diastolic pressure and improves coronary perfusion by increasing blood flow to the arteries. Deflation of the balloon just before systole reduces afterload at the time of systolic contraction. This action facilitates emptying of the left ventricle and improves cardiac output. The balloon catheter is attached to a pump console, which is triggered by an ECG tracing and arterial waveform. In patients undergoing high-risk percutaneous coronary intervention (PCI) or those at risk for cardiogenic shock, a percutaneous ventricular assist device may be used. These devices are used temporarily to decrease the myocardial workload and oxygen consumption of the heart and increase cardiac output and peripheral perfusion. Immediate reperfusion is an invasive intervention that shows some promise for managing cardiogenic shock. The patient is taken to the cardiac catheterization laboratory, and an emergency left-sided heart catheterization is performed. If he or she has a treatable occlusion or occlusions, the interventional cardiologist performs a PCI in the catheterization laboratory, or the patient is transferred to the operating suite for a coronary artery bypass graft (CABG) Managing Right Ventricular Failure - In about a third of patients with inferior MIs, right ventricular infarction and failure develop. In this instance, the right ventricle fails independently of the left. - Decreased cardiac output with a paradoxical pulse, clear lungs, and jugular venous distention occurs when the patient is in semi-Fowler position. - The desired outcome of management is to improve right ventricular stroke volume by increasing right ventricular fiber stretch or preload. - To enhance right ventricular preload, give sufficient fluids to increase right atrial pressure to 20 mm Hg - If medical therapy is not sufficient to support the right ventricle and reverse the shock state, a right percutaneous ventricular assist device may be needed. This is a temporary measure to support the failing heart while treating the cardiogenic shock with medical therapy. Coronary Artery Bypass Graft Surgery (CABG) - Improve blood flow to myocardial tissue at risk for ischemia or infarction resulting from occlusion of the artery, if medical regimen not successful - It is the most common type of cardiac surgery and the most common procedure for older adults. Almost half of all CABGs are done for patients older than 65 years. - The occluded coronary arteries are bypassed with the patient’s own venous or arterial blood vessels or synthetic grafts. The internal thoracic artery (also referred to as the internal mammary artery [IMA]) is often the graft of choice because it has an excellent patency rate many years after the procedure. - CABG is indicated when patients do not respond to medical management of CAD or when disease progression is evident - Candidates for surgery are patients who have: o o o o o o o Angina with greater than 50% occlusion of the left main coronary artery that cannot be stented Unstable angina with severe two-vessel disease, moderate three-vessel disease, or small-vessel disease in which stents could not be introduced Ischemia with heart failure Acute MI with cardiogenic shock Signs of ischemia or impending MI after angiography or percutaneous coronary intervention Valvular disease Coronary vessels unsuitable for PCI Preop Care: Standard preop teaching. Familiarize the client and family with the cardiac critical care unit. Inform the client to expect a sternal incision, possible arm or leg incision(s), one or two chest tubes, a Foley catheter, and several IV fluid catheters. ET tube so they will be unable to speak. Will be on ventilator, will need to breathe with the ventilator and not fight it. Pain meds will be available. Medications may be discontinued preop (usually, diuretics 2 to 3 days before surgery, digoxin 12 hours before surgery, and aspirin and anticoagulants 1 week before surgery). Postop Care: ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Manage F&E balance Complications—hypotension, hypothermia, hypertension, bleeding, cardiac tamponade, change in level of consciousness Administer medications as prescribed, which may include potassium chloride, antihypertensives, antidysrhythmics, and antibiotics. The patient should report any pain to the nursing staff Most of the pain will be in the site where the vessel was harvested. (With the use of endovascular vessel harvesting [EVH] and one or two small incisions, the pain and edema are less than for previously performed procedures.) Analgesics will be given to decrease pain Coughing and deep breathing are essential to prevent pulmonary complications Early ambulation is important to decrease the risk for venous thrombosis and possible embolism All CABG patients, especially those with IMA grafts, are at high risk for atelectasis, the number-one complication. Heart-lung bypass circuitry used during cardiopulmonary bypass - - - - - - Cardiopulmonary bypass (CPB) is used to provide oxygenation, circulation, and hypothermia during induced cardiac arrest. Blood is diverted from the16 heart to the bypass machine, where it is heparinized, oxygenated, and returned to the circulation through a cannula placed in the ascending aortic arch or femoral artery During bypass, the patient’s core temperature remains between 95°F (35°C) (cold cardioplegia) and normal temperature (warm cardioplegia). Although cooling decreases the rate of metabolism and demand for oxygen, keeping the heart “warm” decreases postoperative complications that were more common when cold cardioplegia was used. The heart is perfused with a potassium solution, which decreases myocardial oxygen consumption and causes the heart to stop during diastole. This process ensures a motionless operative field and prevents myocardial ischemia. Once the heart is arrested, the grafting procedure can begin. The surgeon uses the internal mammary artery (IMA), a saphenous vein, and/or a radial artery to bypass blockages in the coronary arteries. The distal end of the vessel graft is dissected and attached below the clot in the coronary artery. If the surgeon uses a venous graft or the radial artery, it is anastomosed (sutured) proximally to the aorta and distally to the coronary artery just beyond the occlusion, thus improving myocardial perfusion. After flow rates through the grafts are measured, the heart is rewarmed slowly. The cardioplegic solution is flushed from the heart. The heart regains its rate and rhythm, or it may be defibrillated to return it to a normal rhythm. When the procedure is completed, the patient may be rewarmed (if cold cardioplegia was used) and weaned from the bypass machine while the grafts are observed for patency and leakage. The surgeon may place atrial and ventricular pacemaker wires and mediastinal and pleural chest tubes. Finally the surgeon closes the sternum with wire sutures. Two methods of coronary artery bypass graft Minimally invasive direct coronary artery bypass. The minimally invasive direct coronary artery bypass (MIDCAB) may be indicated for patients with a lesion of the left anterior descending (LAD) artery. In the most common MIDCAB procedure, a left thoracotomy incision is made, and the rib retraction is required. Then the left internal mammary artery (IMA) is dissected and attached to the still-beating heart below the level of the lesion. Cardiopulmonary bypass (CPB) is not required. Endovascular (endoscopic) vessel harvesting. Regardless of whether the traditional CABG or the MIDCAB is performed, the donor vessel may be obtained using an endoscope rather than a large surgical incision. The radial artery or a vein in the leg may be taken with this method. Instead of a large, painful incision, the patient has one or two very small incisions in the leg or arm. This procedure has decreased hospital length of stay, postoperative complications, and pain. Off-pump coronary artery bypass Off-pump coronary artery bypass (OPCAB) is a procedure in which open-heart surgery is performed without the use of a heart-lung bypass machine. Advantages include shorter hospital stays and decreased mortality rate, risk for infection, and cost. The disadvantage of OPCAB is that it requires cardiac surgeons to have increased skill to master the technique. Robotic-assisted heart surgery Less invasive open-heart surgery. Surgeons operate endoscopically through very small incisions in the chest wall. Simplifies the surgical process, eliminates tremors that can exist with human hands, increases the ability to reach otherwise inaccessible sites, and improves depth perception and visual acuity. Shorter hospital stays (average stay is 2 to 3 days), less pain because of smaller incisions, no need for heart-lung bypass machine, less anxiety for the patient, and greater patient acceptance. Care Coordination and Transition Management Home Care Management - - Patients who have experienced a myocardial infarction (MI), angina, or coronary artery bypass graft (CABG) surgery are usually discharged to home or to a transitional care setting with drug therapy and specific activity prescriptions. Patients should not be discharged to home alone. Assess whether the patient has family or friends to provide assistance. In some cases, a home care nurse may be needed Cardiac rehabilitation is available in most communities for patients after an MI or CABG surgery, but only a small percentage participate in structured rehabilitation programs Self-Management Education - Develop a teaching plan, which usually includes education about the normal anatomy and physiology of the heart, the pathophysiology of angina and MI, risk factor modification, activity and exercise protocols, cardiac drugs, and when to seek medical assistance. Risk Factor Modification - Modifications may include tobacco cessation, altered dietary patterns, regular exercise, BP control, and blood glucose control. - The mainstays of cholesterol control are nutritional therapy and antihyperlipidemic agents - Maintain adequate dietary potassium, calcium, and magnesium intake. - Collaborate with the cardiac rehabilitation specialist to establish an activity and exercise schedule as part of rehabilitation, depending on the cardiac procedure that was performed - Instruct the patient to remain near home during the first week after discharge and to continue a walking program. Patients may engage in light housework or any activity done while sitting and that does not precipitate angina. - During the second week, they are encouraged to increase social activities and possibly to return to work part-time. By the third week, they may begin to lift objects as heavy as 15 lb but should avoid lifting or pulling heavier objects for the first 6 to 8 weeks - Patients may begin a simple walking program by walking 400 feet twice a day at the rate of 1 mile/hr the first week after discharge and increasing the distance and rate as tolerated, usually weekly, until they can walk 2 miles at 3 to 4 miles/hr. - After a limited exercise tolerance test, the cardiac rehabilitation specialist or nurse encourages the patient to join a formal exercise program, ideally one that helps him or her monitor cardiovascular progress. The program should include 5- to 7-minute warm-up and cool-down periods and 30 minutes of aerobic exercise. The patient should engage in aerobic exercise a minimum of three (and preferably five) times a week. Complementary and Integrative Health. - Techniques such as progressive muscle relaxation, guided imagery, music therapy, pet therapy, and therapeutic touch may decrease anxiety, reduce depression, and increase adherence with activity and exercise regimens after heart surgery. - Teach patients that adding omega-3 fay acids from fish and plant sources has been effective for some patients in reducing lipid levels, stabilizing atherosclerotic plaques, and reducing sudden death from an MI. - Patients often take a number of other supplements, such as vitamin E, coenzyme Q10, Pantesin, and vitamin B complex to decrease the risk for heart disease. However, studies do not show that these substances are helpful in reducing coronary artery disease. Sexual Activity. - Inform the patient and his or her partner that engaging in their usual sexual activity is unlikely to damage the heart Patients can resume sexual intercourse on the advice of the health care provider, usually after an exercise tolerance assessment In general, those who can walk one block or climb two flights of stairs without symptoms can usually safely resume sexual activity Drug Therapy. - Assess patients with diabetes mellitus for their ability to control hyperglycemia - Teach patients that some medications, such as beta blockers, may block symptoms of hypoglycemia. - Many patients with angina are discharged while taking aspirin, a beta blocker, a calcium channel blocker, a statin agent, and a nitrate. Those who have experienced an MI may require dual antiplatelet therapy with aspirin and a P2Y12 inhibitor, a beta blocker, a statin drug, and, if the ejection fraction is below 40%, an ACEI and/or an ARB. - It is recommended that all patients with cardiovascular disease receive an annual influenza vaccine and that patients over age 56 also receive the pneumococcal vaccine Health Care Resources - The American Heart Association (AHA) is an excellent source for booklets, films, CDs, DVDs, cookbooks, and professional service referrals for the patient with coronary artery disease (CAD). - Many shopping malls open before shopping hours to allow a measured walking program indoors. This opportunity is particularly popular with older patients because it provides a good support group and allows for an appropriate place to exercise in inclement weather - Mended Hearts is a nationwide program with local chapters that provides education and support to coronary artery bypass graft (CABG) patients and their families. - Smoking-cessation programs and clinics and weight-reduction programs are located within the community. Many hospitals and places of worship also sponsor health fairs, BP screening, and risk-factor modification programs. Evaluation: Evaluate Outcomes ◦ Expected outcomes are that patient will: ◦ ◦ ◦ State that pain is alleviated Have adequate myocardial perfusion Be free of complications such as dysrhythmias and heart failure Chapter 15 – Concepts of Infusion Therapy Key Terms adverse drug events (ADEs) An unintended harmful reaction to an administered drug ambulatory pumps Infusion therapy pump generally used with a home care patient to allow a return to his or her usual activities while receiving infusion therapy catheter-related bloodstream infection (CRBSI) Health care−acquired bloodstream infections caused by the presence of any type of intravenous catheter catheter-related bloodstream infection (CRBSI) prevention bundle A nationally recognized set of evidence-based practices to prevent CRBSIs central line−associated bloodstream infection (CLABSI) Health care−acquired bloodstream infections caused by the presence of a central intravenous line compartment syndrome A condition in which increased tissue pressure in a confined anatomic space causes decreased blood flow to the area, leading to hypoxia and pain extravasation Escape of fluids or drugs into the subcutaneous tissue; a complication of intravenous infusion therapy. implanted port A surgically implanted vascular access device (VAD) where the port is placed in a subcutaneous pocket; used for long-term or frequent infusion therapy. infiltration Leakage of IV solution into the tissues around the vein. infusate Solution that is infused into the body infusion therapy Delivery of parenteral medications and fluids through a variety of catheter types and locations using multiple techniques and procedures, such as intravenous therapy to deliver solutions into the vascular system midline catheters A VAD that is 3 to 8 inches long and inserted through the veins of the antecubital fossa. nontunneled central venous catheter (CVC) A multilumen VAD inserted through the subclavian or jugular vein using sterile technique peripheral IV therapy IV therapy in which a vascular access device (VAD) is placed in a peripheral vein, usually in the arm peripherally inserted central catheter (PICC) A long VAD inserted through a vein at the antecubital fossa phlebitis Inflammation of a vein that can predispose patients to thrombosis short peripheral catheter (SPC) A VAD composed of a plastic cannula, built around a sharp stylet for venipuncture, which extends slightly beyond the cannula and is advanced into the vein secondary (piggyback) administration set A short tubing set that is attached to the primary administration set and used to deliver intermittent medications smart pumps Infusion pumps with dosage calculation software syringe pumps Pump for infusion therapy that uses a battery-powered piston to push the plunger continuously at a selected rate; limited to small-volume or intermittent infusions. thrombophlebitis Presence of a thrombus associated with inflammation thrombosis Formation of a blood clot within a blood vessel tunneled central venous catheter A surgically implanted VAD used for long-term infusion therapy in which the catheter lies in a subcutaneous tunnel, separating the points where the catheter enters the vein from where it enters the skin vascular access device (VAD) An infusion catheter placed in a blood vessel to deliver fluids and medications vesicant medications Drugs that cause severe tissue damage if they escape into the subcutaneous tissue; also referred to as vesicants. Infusion Therapy - The delivery of medications in solution or fluids by a parenteral route, which requires piercing of the skin with a needle or catheter - Intravenous (IV) therapy is the most common route - The most common reasons for using infusion therapy are o Maintain fluid balance or correct fluid imbalance o Maintain electrolyte or acid-base balance or correct electrolyte or acidbase imbalance o Administer medications o Replace blood or blood products Types of Infusion Therapy Fluids Normal serum osmolarity (adults) = 270 to 300 mOsm/L IV solutions (including parenteral nutrition) Isotonic = 270 to 300 mOsm/L Hypertonic = Fluids >300 mOsm/L Hypotonic = Fluids <270 mOsm/L - - - - - - When an isotonic infusate (solution that is infused into the body) is used, water does not move into or out of the body’s cells and remains in the extracellular compartments. Therefore patients, especially older adults, receiving isotonic solutions are at risk for fluid overload Hypertonic solutions are used to correct altered fluid and electrolyte balance and acid-base imbalances by moving water out of the body’s cells and into the interstitial spaces and bloodstream. Electrolytes and other particles also move across cell membranes across a concentration gradient (from higher concentration to lower concentration). Parenteral nutrition solutions are hypertonic Hypotonic infusates move water into cells to expand them Patients receiving either hypertonic or hypotonic fluids are at risk for phlebitis and infiltration. o Phlebitis is the inflammation of a vein caused by mechanical, chemical, or bacterial irritation. o Infiltration occurs when IV solution leaks into the tissues around the vein. The pH of IV solutions is a measure of acidity or alkalinity and usually ranges from 3.5 to 6.2. Extremes of both osmolarity and pH can cause vein damage, leading to phlebitis and thrombosis (blood clot in the vein). Thus fluids and medications with a pH value less than 5.0 and more than 9.0 and with an osmolarity more than 600 mOsm/L are best infused in the central circulation where greater blood flow provides adequate hemodilution (ex: TPN) Drugs with vasoconstrictive action (e.g., dopamine or chemotherapeutic agents) are vesicants (chemicals that damage body tissue on direct contact) that can cause extravasation Extravasation results in severe tissue integrity impairment as manifested by blistering, tissue sloughing, or necrosis from infiltration into the surrounding tissues Blood and Blood Components - Blood transfusion is given by using packed red blood cells, created by removing a large part of the plasma from whole blood. - Other available blood components include platelets, fresh frozen plasma, albumin, and several specific clotting factors. Most organizations use the International Society of Blood Transfusion (ISBT) universal bar-coding system to ensure the right blood for the right patient. The ISBT system includes four components that must be present on the blood label both in bar code and in eye-readable format (1) a unique facility identifier (2) the lot number relating to the donor (3) the product code (4) the ABO group and Rh type of the donor Drug Therapy - IV drugs provide a rapid therapeutic effect but can lead to immediate serious reactions, called adverse drug events (ADEs). - IV administration also requires knowledge of appropriate dilution, rate of infusion, pH and osmolarity, compatibility with other IV medications, appropriate infusion site (peripheral versus central circulation), potential for vesicant/irritant effects, and specific aspects of patient monitoring because of its immediate effect. Prescribing Infusion Therapy A prescription for infusion therapy written by an authorized primary health care provider (physician, nurse practitioner, or physician assistant) is necessary before IV therapy begins. To be complete, the prescription for infusion fluids should include: o Specific type of fluid to be infused o Rate of administration written in milliliters per hour (mL/hr) or the total amount of fluid and the total number of hours for infusion (e.g., 125 mL/hr or 1000 mL/8 hr) o Specific drugs and dose to be added to the solution such as electrolytes or vitamins A drug prescription should include: o Drug name, preferably by generic name o Specific dose and route o Frequency of administration o Time(s) of administration o Length of time for infusion (number of doses/days) o Purpose (required in some health care agencies, especially nursing homes) Vascular Access Devices - An infusion catheter, also known as a vascular access device (VAD), is a plastic tube placed in a blood vessel to deliver fluids and medications. The location of the VAD, either a peripheral vein or a large central vein in the chest, is determined by the specific type and purpose of the therapy. Types of catheters used for peripheral and central IV therapy Short peripheral catheters Midline catheters Peripherally inserted central catheters (PICC) Nontunneled percutaneous central venous catheters (CVC) Tunneled catheters Implanted ports Hemodialysis catheters Peripheral Intravenous Therapy Short infusion catheters are the most commonly used vascular access devices (VADs) for peripheral IV therapy. They are usually placed in the veins of the arm. Another catheter used for peripheral IV therapy is a midline catheter. Short Peripheral Catheters - Short peripheral catheters are composed of a plastic cannula built around a sharp stylet extending slightly beyond the cannula - The stylet allows for the venipuncture, and the cannula is advanced into the vein. Once the cannula is advanced into the vein, the stylet is withdrawn. - These catheters are designed with a safety mechanism to cover the sharp end of the stylet after it is removed from the patient to decrease the risk of accidental injury. - The stylet is a hollow-bore, blood-filled needle that carries a high risk for exposure to bloodborne pathogens if needlestick injury occurs. Insertion and Placement Methods - Short peripheral catheters are most often inserted into superficial veins of the forearm. - In emergent situations, these catheters can also be used in the external jugular vein of the neck. - Avoid the use of veins in the lower extremities of adults, if possible, because of an increased risk for deep vein thrombosis and infiltration. - Short catheters range in length from ¾ inch to 1¼ inch, with gauge sizes from 26-gauge (the smallest) to 14-gauge (large bore). - Choose the smallest gauge catheter capable of delivering the prescribed therapy with consideration of all the contributing factors, including expected duration, vascular characteristics, and comorbidities - When selecting the site for insertion of a peripheral catheter, consider the patient’s age, history, and diagnosis; the type and duration of the prescribed therapy; and, whenever possible, the patient’s preference. - Vascular visualization technology (e.g., near infrared and ultrasound devices) are now available as tools to assist in IV line placement - Ultrasound-guided peripheral IV insertion can allow insertion into deeper veins. This technology has been shown to be valuable in assisting with cannulation of peripheral veins that the nurse cannot access with sight and touch. - - - Arteries and nerves often lie parallel to deep veins, and training is essential to learn to identify these structures and avoid damaging them. In addition, when deeper veins are used, infiltration may go undetected until a significant amount of fluid has collected in the tissues. For patients who need IV access but are at risk for fluid overload or do not need additional IV fluids, the peripheral vascular access device (VAD) can be converted into an intermittent IV lock, also called a saline lock. This device allows administration of specific drugs given IV push (e.g., furosemide) or on an intermittent basis using a medication administration set. IV antibiotics are frequently administered using a saline lock. The intermittent device is flushed with saline before and after drug administration to ensure patency and prevent occlusion with a blood clot. These VADs are not recommended for obtaining routine blood samples due to the risk of hemolysis. Site Selection and Skin Preparation - - - The most appropriate veins for peripheral catheter placement include the dorsal venous network (i.e., basilic, cephalic, and median veins and their branches) However, cannulation of veins on the hand is not appropriate for older patients with a loss of skin turgor and poor vein condition or for active patients receiving infusion therapy in an ambulatory care clinic or home care. Use of veins on the dorsal surface of the hands should be reserved as a last resort for short term infusion of nonvesicant and nonirritant solutions in young patients. Mastectomy, axillary lymph node dissection, lymphedema, paralysis of the upper extremity, and the presence of dialysis grafts or fistulas alter the normal pattern of blood flow through the arm. Using veins in the extremity affected by one of these conditions requires a primary health care provider’s order Aseptic skin preparation and technique before IV insertion are crucial Catheter-related bloodstream infection (CRBSI) can occur from a peripheral IV site. Both the CDC and the INS have best practice guidelines developed to prevent infection Midline Catheter - Anywhere from 3 to 8 inches long, 3 to 5 Fr, and double or single lumen - They are inserted into a vein of the upper arm - Midline catheters reduce the number of repeated IV cannulations, which reduces patient discomfort, increases patient satisfaction, and contributes to organizational efficiency Indications for midline catheters include fluids for hydration and drug therapy that are given longer than 6 days and up to 14 days Midline catheters should not be used for infusion of vesicant medications—drugs that cause severe tissue damage if they escape into the subcutaneous tissue (extravasation). There is concern that at a midline tip location, larger amounts of the drug may extravasate before the problem is detected Central Intravenous Therapy - - In central IV therapy the vascular access device (VAD) is placed in the central circulation, specifically within the superior vena cava (SVC) near its junction with the right atrium, also called the caval-atrial junction (CAJ). A number of types of central vascular access devices (CVADs) are available, depending on the purpose, duration, and insertion site availability Peripherally Inserted Central Catheters (PICC) - A long catheter inserted through a vein of the antecubital fossa (inner aspect of the bend of the arm) or the middle of the upper arm. - In adults the PICC length ranges from 18 to 29 inches (45 to 74 cm), with the tip residing in the superior vena cava (SVC) ideally at the caval-atrial junction (CAJ - Before the catheter can be used for infusion, a chest x-ray indicating that the tip resides in the lower SVC is required when the catheter is not placed under fluoroscopy or with the use of the electrocardiogram tip-locator technique. Nontunneled Percutaneous Central Venous Catheters - Inserted through subclavian vein in upper chest or jugular veins in neck 7 to 10 inches (15 to 25 cm) long; up to 5 lumens Tip resides in superior vena cava Short term use Tunneled Central Venous Catheters - - Tunneled central venous catheters are VADs that have part of the catheter lying in a subcutaneous tunnel, separating the points where the catheter enters the vein from where it exits the skin. The catheter has a cuff made of a rough material that is positioned inside the subcutaneous tunnel. These cuffs commonly contain antibiotics, which also reduce the risk for infection Single, dual, and triple lumens are available. These catheters were originally named for the physicians who designed them, including Broviac, Hickman, and Leonard catheters. Tunneled catheters are used primarily when the need for infusion therapy is frequent and long term. Tunneled catheters are chosen when several weeks or months of infusion therapy are needed and a PICC is not a good choice Implanted Ports - This type of device is chosen for patients who are expected to require IV therapy for more than a year Implanted ports consist of a portal body, a dense septum over a reservoir, and a catheter They can be single or double lumen and come in various sizes A subcutaneous pocket is surgically created to house the port body. The catheter is inserted into the vein and attached to the portal body. The septum is made of self-sealing silicone and is located in the center of the port body over the reservoir; the catheter extends from the side of the port body. The incision is closed, and no part of the catheter is visible externally; therefore this device has the least impact on body image Hemodialysis Catheters - Hemodialysis catheters have very large lumens to accommodate the hemodialysis procedure or a pheresis procedure that harvests specific blood cells - They may be tunneled for long-term needs or nontunneled for short-term needs - CRBSIs and vein thrombosis are common complications; therefore this catheter should not be used for administration of other fluids or drugs except in an emergency. - The generalist RN generally does not access hemodialysis catheters. These are maintained by specially trained hemodialysis nurses. Infusion Systems Containers - Infusion containers are made of glass or plastic - For plastic containers – concern of exposure to PVC and DEHP Administration Sets - The administration set is the connection between the catheter and the fluid container. - Some sets are generic, meaning that they are appropriate for most infusions. - Other sets are used for specific types of infusions such as blood transfusion. - Still others are dedicated, meaning that they must be used with a specific manufacturer’s infusion controlling device Secondary Administration Sets - A primary continuous administration set is used to infuse the primary IV fluid by either a gravity infusion or an electronic infusion pump. A short secondary administration set, also known as a piggyback set, is attached to the primary set at a Y–injection site and is used to deliver intermittent medications - Primary and secondary continuous infusion administration sets used to infuse fluids other than parenteral nutrition and lipids can be used for up to 96 hours unless the closed system has been compromised Intermittent Administration Sets - When no primary continuous fluid is being infused, use an intermittent administration set to infuse multiple doses of medications through a catheter that has been capped with a needleless connection device. - Because both ends of the set are being manipulated with each dose, the INS standards of practice state that this set should be changed every 24 hours Add-on Devices - - Several other types of add-on devices include short extension sets, injection caps, and filters. Administration sets have two ways to connect to the catheter hub: a slip lock or a Luer-Lok. The slip lock is a male end that slips into the female catheter hub. A Luer-Lok connection has the same male end with a threaded collar that requires twisting onto the corresponding threads of the catheter hub Filters may be part of the administration set or separate add-on pieces. Their purpose is to remove particulate mater, microorganisms, and air from the infusion system. Filters should be placed as close to the catheter hub as possible Needleless Connection Devices - Needlestick Safety and Prevention Act. This regulation requires the use of devices engineered with safety mechanisms and mandates that staff who perform these tasks be directly involved with selecting products. It also requires each employer to maintain a sharps injury log with details of each incident. Many products are designed to minimize health care workers’ exposure to contaminated needles. Luer-lock–activated devices are the most common design for needleless systems today - it is imperative that the connector be disinfected with alcohol or chlorhexidine/alcohol before and after each use with a vigorous scrub for 5 to 60 seconds Rate-Controlling Infusion Devices - The ability to regulate the rate and volume of infusions is critical to the safe and accurate administration of medications and fluids to patients - Syringe pumps use an electronic or battery-powered piston to push the plunger of a large syringe inserted into the pump mechanism continuously at a selected milliliter-per-hour rate. The use of syringe pumps is limited to smallvolume continuous or intermittent infusions and depends on the syringe size. Antibiotics and patient-controlled analgesia are frequently delivered with syringe pumps. Patients requiring fluid restrictions can also benefit from using a syringe pump because smaller yet accurate volumes can be used to dilute medications - Ambulatory pumps are generally used for home care patients and allow them to return to their usual activities while receiving infusion therapy. These pumps have a wide range of sizes, with some requiring a backpack, but they usually weigh less than 6 lb. They are typically used to accurately deliver continuous infusions such as parenteral nutrition, pain medication, and many programmable drug schedules. - In the past few years, smart pumps (infusion pumps with dosage calculation software) have been promoted to reduce adverse drug events (ADEs). Multiple libraries of drug information are stored in the pump manufacturer’s medical management system. This software allows the facility to preprogram dosing limits, especially for high alert drugs Nursing Care For Patients Receiving Intravenous Therapy Educating the Patient - Before catheter insertion, educate the patient and family about: o The type of catheter to be used o Hand hygiene and aseptic technique for care of the catheter o The therapy required o Alternatives to the catheter and therapy o Activity limitations o Any signs or symptoms of complications that should be reported to a health care professional Performing the Nursing Assessment - All central VADs require documentation of tip location at the caval-atrial junction (CAJ) by electrocardiogram technology, fluoroscopy, or chest x-ray - Nursing assessment for all infusion systems should be systematic beginning at the insertion site, working upward following the tubing to the infusion bag. Know the type of catheter your patient has in place. Be sure to find out the length of catheter, the insertion site, and tip location to perform a complete assessment. - Assess the insertion site by looking for redness, swelling, hardness, or drainage. Also assess the skin underneath the dressing, especially for signs of medical adhesive–related skin injury - When a midline catheter or PICC is used, assess the entire extremity and upper chest for signs of phlebitis and thrombosis - When a tunneled catheter is used, assess the exit site, the entire length of the tunnel, and the point where the catheter enters the vein - For implanted ports, assess the incision and surgically created subcutaneous pocket - Assess the integrity of the dressing, making sure that it is clean, dry, and adherent to the skin on all sides - Check all connections on the administration set and ensure that they are secure. Be sure that they are not taped. - Check the rate of infusion for all fluids by either counting drops or checking the infusion pump. - Check all labels on containers for the patient’s name and fluid or medication. Be sure that the correct solution is being infused! Securing and Dressing the Catheter - Securing the catheter is an important step in the prevention of complications. Tape, sutures, and specially designed securement devices can be used for this purpose. - the StatLock IV stabilization device prevent peripheral and central catheters from becoming dislodged. They prevent complications such as phlebitis and infiltration. To prevent skin tears, remove the adhesive on a StatLock with 70% alcohol. - PICCs and nontunneled central catheters may be sutured in place; however, this creates additional breaks in the skin that could become infected\ - Sterile dressings used over the insertion site protect the skin and puncture site - Change tape and gauze dressings every 48 hours; change transparent membrane dressings, such as Tegaderm, every 5 to 7 days - Never pull it off by pulling away from the insertion site because this could dislodge the catheter! - After removing the dressing from a midline catheter or any central venous catheter, note the external catheter length. Compare this length with the original length at insertion. Changing Administration Sets and Needleless Connectors - Plan the change of administration sets and fluid containers to occur at the same time, if possible, to minimize the number of times the system is opened. - Needleless connector devices can be changed when the administration set is changed. If it is being used for intermittent infusions, the device should be changed at least once per week - Precautions to prevent air emboli are required when changing the set or connectors attached to any catheter; however, central venous catheters require special attention. Most catheters have a pinch clamp that should be closed during this procedure. - Techniques used to increase the intrathoracic pressure and prevent air embolism during IV set change include: o o o o Placing the patient in a flat or Trendelenburg position to ensure that the catheter exit site is at or below the level of the heart Asking the patient to perform a Valsalva maneuver by holding his or her breath and bearing down Timing the IV set change to the expiratory cycle when the patient is spontaneously breathing Timing the IV set change to the inspiratory cycle when the patient is receiving positivepressure mechanical ventilation Controlling Infusion Pressure - In order for fluid to flow through the system, the pressure on the external side must be greater than the pressure at the catheter tip - Fluid flow can be slowed or obstructed from the catheter tip impinging on the vein wall, a thrombus distal to the catheter, or a venous spasm. - Inside the catheter lumen, resistance is created by the catheter length and diameter or deposits of fibrin, thrombus, or drug precipitate Flushing the Catheter - Catheter flushing prevents contact between incompatible drugs and maintains patency of the lumens. - Normal saline alone or normal saline followed by heparinized saline may be used, depending on the device recommendations. Obtaining Blood Samples From Central Venous Catheters - Short peripheral catheters should not be used routinely for obtaining blood samples. - The risks associated with obtaining blood samples from a central venous catheter are numerous. This procedure requires additional hub manipulation, which is a major cause of CRBSI - If blood sampling from a central venous catheter is the best alternative, vigorous cleaning of the connections with 70% alcohol is necessary. - Vacuum tubes attached via a “vacutainer” to the catheter hub eliminate the need to transfer the blood from a syringe into the tubes and therefore do not require exposed needles - After blood draw from any catheter, a flush of 10 to 20 mL sterile normal saline is necessary to ensure a patent line. Be sure to clear the line and cap of blood to prevent a breeding ground for infection. Removing the Vascular Access Device - Lift opposite sides of the transparent dressing and pull laterally to remove the dressing from the site while stabilizing the catheter - Slowly withdraw the catheter from the skin and immediately cover the puncture site with dry gauze - Hold pressure on the site until hemostasis is achieved - Assess the catheter tip to make sure that it is intact and completely removed - Document the time of catheter removal and appearance of the IV site. - If you feel resistance, always stop and never apply force to the catheter. Extreme traction or force could cause the catheter to break and embolize (travel) to the heart or pulmonary circulation - - - Apply heat; allow time for the vein wall to relax. Keeping the extremity warm and dry and asking the patient to drink warm liquids could facilitate removal. Use of medications to relax the vein wall may be required if the catheter cannot be removed after several hours. Nontunneled percutaneous central catheters are removed by removing the dressing as noted above, clipping any sutures, and gently withdrawing the catheter in short segments To prevent venous air embolism when removing any central venous catheter (including PICCs), position the patient in a flat supine or Trendelenburg position according to agency policy. To ensure that the intrathoracic pressure is higher than atmospheric pressure, have the patient hold his or her breath or perform a Valsalva maneuver during removal. If the patient is mechanically ventilated, time the removal to the delivery of an inhalation breath by the ventilator. Be sure to keep the catheter clamped during this procedure. When a central venous catheter is removed, a tract between the skin and vein may create a conduit that could allow air to be pulled into the vein, causing a venous air embolism. After removal, measure the catheter length and compare it with the length documented on insertion. If the entire catheter length was not removed, contact the primary health care provider immediately as this constitutes a medical emergency! Documenting Intravenous Therapy - Be sure to document after insertion of a vascular access device (VAD) and throughout the course of the therapy. - When inserting a venous catheter, remember to document the following: o Date and time of the VAD insertion o Name of the nurse (you) who inserted the VAD o Vein/location that was used for insertion o Type of VAD used including gauge and length of catheter o Number of insertion attempts and locations of attempts before successful insertion o Response of the patient to the VAD insertion process o Type of dressing applied o Type of securement device, if used o Special barrier precautions used, if any o Patient and family education provided related to IV therapy Complications of Intravenous Therapy - Complications from IV therapy can be minor and limited or life threatening. Serious life-altering or life-threatening complications are dramatically increasing in frequency and severity, and present a tremendous financial burden to the U.S. health care system. Catheter-Related Bloodstream Infection - Catheter-related bloodstream infection (CRBSI) is one of the most serious problems, often resulting in patient death. These infections are more common in patients with central VADs but can also occur with peripheral catheters. - CRBSI are one of several preventable hospital-acquired infections (HAIs). Other Complications of Intravenous Therapy - Local complications of IV therapy occur at or near the catheter. - Systemic complications of IV therapy involve the entire vascular system or multiple systems. - For central venous catheters (CVCs), complications can occur during the insertion procedure or during the dwell time Intravenous Therapy and Care of the Older Adult - The aging process causes numerous changes in all body functions, and yet aging occurs differently in each person. Nutrition, environment, genetics, social factors, and education are just a few of the factors that influence the older adult’s needs. Because all body functions are affected, IV therapy can be affected by these changes. Skin Care - Aging skin becomes thinner and loses subcutaneous fat, decreasing the skin’s ability for thermal regulation. - Fewer nerve endings may alter the way an individual experiences pain and other sensations. - Older patients may not perceive acute pain from traumatic venipuncture and may tolerate probing or multiple attempts. However, this action increases the risk for fluid leakage and subsequent infiltration or extravasation injury. Inserting and removing a catheter and dressing could tear the skin layers. - Skin antisepsis is extremely important because of the decreased immunity seen as part of the aging process. - Lipids are normally found in skin as a protective agent, and alcohol easily dissolves lipids. Although greater numbers of organisms may be killed, the skin can also become excessively dry and cracked. - Current recommendations call for using friction when cleaning the skin to penetrate the layers of the epidermis. However, excessive friction may damage fragile skin and cause impaired tissue integrity. - Chlorhexidine is the preferred agent, and the product currently available contains alcohol. - Check for allergies to iodine before using iodine or iodophors. Iodophors such as povidoneiodine require contact with the skin for a minimum of 2 minutes to be effective. - All antiseptic solutions must be thoroughly dry before applying the dressing or tape to ensure adequate adherence to the skin. - Skin should never be shaved before venipuncture as shaving causes microabrasions that can lead to infection. Shaving can also more easily nick the thin, delicate skin of an older adult. If - - necessary, excessive amounts of hair should be clipped to ensure the bandage adheres to the skin. Skin and tissue integrity can easily be compromised by the application of tape or dressings. The use of skin protectant solutions puts a protective barrier between the skin and dressing and improves the adherence of the dressing to the skin. Removal of tape and dressings may require adhesive remover solutions, or an alcohol pad may accomplish the same purpose. Securement devices such as the StatLock require the use of a skin protectant (e.g., Skin- Prep) before applying the device. The protectant prevents skin tearing when the device is removed. Vein and Catheter Selection - Choose insertion sites carefully after considering the patient’s skin integrity, vein condition, and functional ability. - The general principle of starting with the most distal sites usually indicates use of hand veins. However, avoid fragile skin and small, tortuous veins on the back of the hand (dorsum); select the initial IV site higher on the arm - Venous distention must be accomplished with a flat tourniquet; however, the veins may require longer to adequately distend - Avoid hard, cordlike veins. - Blood pressure cuffs can also be used for venous distention. Inflate the cuff and release until the pressure is slightly less than diastolic pressure. - Other methods to distend veins include: o Tapping the vein lightly, but avoiding forceful slapping o Asking the patient to open and close the fist so the muscles can force blood into the veins, making sure that the hand is relaxed when the venipuncture is attempted o Placing the extremity lower than the heart o Applying warm compresses or a heating pad (be careful not to make it too hot) to the entire extremity for 10 to 20 minutes and removing just before making the venipuncture - As with all patients, venipuncture technique requires adequate skin and vein stabilization during the puncture and complete catheter advancement - Veins of an older adult are more likely to roll away from the needle as veins become firmer and more difficult to puncture with aging - Low angles of 10 to 15 degrees between the skin and catheter will improve success with venipuncture. - Choosing a midline catheter or PICC may be best in older patients with poor skin turgor; limited venous sites; or veins that are fragile, tortuous, or hard. These catheters are placed in the upper extremity where venous distention techniques can be used. - Inserting nontunneled percutaneous central catheters in older adults can be much more challenging. Venous distention for insertion requires the Trendelenburg position and a wellhydrated patient to ensure that the veins are adequately distended. Cardiac and Renal Changes - Because of changes in cardiac and renal status in older adults, the accuracy of infusion volume and flow rate measurements is very important in the older adult. Older adults are more prone to fluid overload with resulting heart failure or dehydration with subsequent poor perfusion - When fluid restrictions are required, medications could be diluted in small quantities and delivered using a syringe pump or a manual IV push. Consult with a pharmacist to determine the - smallest amount of diluent required. This alternative may allow the patient to have more fluid to drink. Serum sodium levels should be considered when normal saline is routinely used for dilution in patients with hypertension or cardiac problems An increasing number of patients with chronic illness require repeated and frequent IV therapies. Many of these patients are vein depleted and need vein preservation. Subcutaneous and intraosseous routes have demonstrated effectiveness in emergency resuscitation. These procedures may also be beneficial for routine infusion of isotonic, nonirritant, nonvesicant solutions in patients with chronic illness and vein depletion Subcutaneous Infusion Therapy - Subcutaneous infusion therapy has been used for a variety of drug infusions. Most commonly it is used for administration of pain medications and insulin therapy. It is beneficial for palliative care patients who cannot tolerate oral medications, when IM injections are too painful, or when vascular access is not available or is too difficult to obtain. - Hypodermoclysis or “clysis” involves the slow infusion of isotonic fluids into the patient’s subcutaneous tissue for the purpose of slow, steady rehydration. - Hypodermoclysis can be used for short-term fluid volume replacement. - The patient must have sufficient sites of intact skin without infection, inflammation, bruising, scarring, or edema. The most common sites are the front and sides of the thighs and hips, the upper abdomen, and the area under the clavicle. Unlike IV therapy, the upper extremities should not be used because fluid is absorbed more readily from sites with larger stores of adipose tissue. - Hypodermoclysis is not appropriate for emergency resuscitations and should not be used for high quantity or emergent fluid replacement needs. - Hyaluronidase, an enzyme that improves the absorption of the infusion, may be prescribed by the primary health care provider and is mixed with each liter of infusion fluid. - A small-gauge (25 to 27) winged infusion or “butterfly” needle, a small gauge short peripheral catheter, or an infusion set specially designed for subcutaneous infusion can be chosen. - When choosing the infusion site, consider the patient’s level of activity. The area under the clavicle or the abdomen prevents difficulty with ambulation. - Clip excess hair in the area and clean the chosen site with the antiseptic solution, preferably 2% chlorhexidine gluconate in 70% isopropyl alcohol to prevent infection. - Prime the infusion tubing and the attached subcutaneous infusion set or winged needle. - Gently pinch an area of about 2 inches (5 cm) and insert the needle using sterile technique. - After securing the needle, cover the site with a transparent dressing. - Assess the site every 4 hours while in a hospital setting and at least twice daily while at home. Redness, warmth, leakage, bruising, swelling, and reports of pain indicate tissue irritation and possible impaired tissue integrity. - If these symptoms occur, remove the infusion needle. Rotate the site at least once a week. - Other complications include pooling of the fluid at the insertion site and an uneven fluid drip rate. Both of these problems may be resolved by restarting the infusion in another location. An infusion pump may also be used. Small ambulatory infusion pumps can be used to allow for greater mobility. • • Usually for pain medication, insulin therapy Benefits • Patients undergoing palliative care who cannot tolerate oral medications • When IM injections are too painful, or when vascular access is not available or difficult to obtain Intraosseous Infusion Therapy - Intraosseous (IO) therapy allows access to the rich vascular network in the red marrow of bones. - Victims of trauma, burns, cardiac arrest, diabetic ketoacidosis, and other life-threatening conditions benefit from this therapy because health care providers often cannot access these patients’ vascular systems for traditional IV therapy - The IO route is for short-term therapy and should be used only during the immediate period of resuscitation and should not be used longer than 24 hours. After establishing access, efforts should continue to obtain IV access as well. - There are few contraindications for IO infusion. The only absolute contraindication is fracture in the bone to be used as a site. Conditions such as severe osteoporosis, osteogenesis imperfecta, or other conditions that increase the risk for fracture with insertion of the IO needle and skin infection over the site may also be contraindications for some patients. - Repeated attempts to access the same site should be avoided. - Any needle can be used to provide IO therapy and access the medullary space (marrow). However, 15- or 16-gauge needles specifically designed for IO therapy are preferred. - New technology using a battery-powered drill has improved the ease of IO insertion. - A number of sites can be used, including the proximal tibia (tibial tuberosity), distal femur, medial malleolus (inner ankle), proximal humerus, and iliac crest. The proximal tibia is the most common site accessed for IO therapy - During insertion, the leg is restrained, and the site cleaned with an antiseptic agent such as chlorhexidine. - After successful insertion the needle must be secured to prevent movement out of the bone. - The same doses of fluids and medications can be infused by IO therapy as IV, and an infusion pump should be used for rapid flow rates. - During the procedure most patients rate the pain as a 2 or 3 on a scale of 0 to 10. Lidocaine 1% is used to anesthetize the skin, subcutaneous tissue, and periosteum to promote comfort. - Improper needle placement with infiltration into the surrounding tissue is the most common complication of IO therapy. Accumulation of fluid under the skin at either the insertion site or on the other side of the limb indicates that the needle either is not far enough in to penetrate the bone marrow or is too far into the limb and has protruded through the other side of the shaft. - Needle obstruction occurs when the puncture has been accomplished but flushing has been delayed. This delay may cause the needle to become blocked with bone marrow. - Osteomyelitis is an unusual but serious complication of IO therapy. You can help prevent this with meticulous aseptic technique, proper hand hygiene, and removal of the catheter as soon as it is no longer needed. - Compartment syndrome is a condition in which increased tissue pressure in a confined anatomic space causes decreased perfusion (peripheral blood flow to the area). The decreased circulation to the area leads to hypoxia and pain in the area. - Although the complication is rare in IO therapy, the nurse should monitor the site carefully and alert the primary health care provider promptly if the patient exhibits any signs of decreased circulation to the limb such as coolness, swelling, moltting, or discoloration. Without improvement in perfusion to the limb, the patient could ultimately require amputation of the limb. • Usually for Trauma, burns, cardiac arrest, DKA, life-threatening conditions • Benefits • Allows access to rich vascular network in the red marrow of bones • Can be used in adults Intra-Arterial Infusion Therapy • Used to obtain repeated arterial blood samples, monitor hemodynamic pressures, infuse chemotherapy or fibrolytics • Used for 3-7 days Intraperitoneal Infusion Therapy • Used to treat ovarian and GI tumors that have moved into the peritoneum • Can be implanted for long-term treatment or used as external for temporary use Intraspinal Infusion Therapy • • Intrathecal medications infused into subarachnoid space and directly into cerebral spinal fluid Used primarily for postoperative and persistent pain Key terms: epistaxis: Nosebleed. gas exchange: Oxygen transport to the cells and carbon dioxide transport away from cells through ventilation and diffusion. hypopnea: Lower than normal respiratory rate and depth insufficient for gas exchange. inspissated secretions: Thickly crusted oral and nasopharyngeal secretions that can cause an upper airway obstruction (also known as mucoid impaction). laryngectomee: An adult who has had a laryngectomy. obstructive sleep apnea (OSA): A breathing disruption during sleep that lasts at least 10 seconds and occurs a minimum of five times in an hour. polysomnography: A formal and definitive overnight sleep study with direct observation of the patient while he or she wears a variety of monitoring equipment to evaluate depth of sleep, type of sleep, respiratory effort, oxygen saturation, carbon dioxide exhalation, and muscle movement. rhinoplasty: Surgical reconstruction of the nose. upper airway obstruction: Interruption of airflow through nose, mouth, pharynx, or larynx. Anatomy of the Nose and Sinuses Upper Airway Obstruction: Assessment: Recognize Cues Assessment: Recognize Cues Airway obstruction requires prompt care to prevent a partial obstruction from progressing to a complete obstruction. Partial obstruction produces general symptoms such as diaphoresis, tachycardia, anxiety, and elevated blood pressure. Persistent or unexplained symptoms must be evaluated even though vague. Diagnostic procedures include chest or neck x-rays, laryngoscope examination, and CT. Observe for hypoxia and hypercarbia, restlessness, increasing anxiety, sternal retractions, a “seesawing” chest motion, abdominal movements, or a feeling of impending doom from air hunger. Use pulse oximetry or end tidal carbon dioxide (EtCO 2 or PEtCO 2 ) for ongoing monitoring of gas exchange . Continually assess for stridor, cyanosis, and changes in level of consciousness. Interventions Assess for the cause of the obstruction. When the obstruction is caused by the tongue falling back or excessive secretions, slightly extend the patient’s head and neck and insert a nasal or an oral airway. Suction to remove obstructing secretions. If the obstruction is caused by a foreign body that cannot be removed by clearing the oral cavity manually, perform abdominal thrusts (Fig. 26.1) Upper airway obstruction may require emergency procedures such as cricothyroidotomy or tracheotomy to improve gas exchange if the object causing the obstruction cannot be removed quickly. When obstruction is not caused by a foreign object, endotracheal intubation may be needed. Laryngoscopy may be performed to determine the cause of obstruction or to remove foreign bodies. Cricothyroidotomy is an emergency procedure performed by emergency medical personnel as a stab wound through the cricothyroid membrane between the thyroid cartilage and the cricoid cartilage (see Fig. 24.3). Any hollow tube—but preferably a tracheostomy tube—can be placed through the opening to hold this airway open until a tracheotomy can be performed. This procedure is used when it is the only way to secure an airway. Another emergency procedure to bypass an obstruction is the insertion of a 14-gauge needle or a very small endotracheal tube directly into the cricoid space to allow airflow into and out from the lungs. Endotracheal intubation is performed by inserting a tube into the trachea via the nose (nasotracheal) or mouth (orotracheal) by a physician, anesthesia provider, or other specially trained personnel. When pharyngeal or laryngeal edema formation is expected or likely, an endotracheal tube is placed before swelling is severe enough to make insertion impossible. Tracheotomy is a surgical procedure and takes about 5 to 10 minutes to perform. It is best performed in the operating room (OR) with the patient under local or general anesthesia but can be performed at the bedside. Local anesthesia is used if there is concern that the airway will be lost during the induction of anesthesia. A tracheotomy is reserved for the patient who cannot be easily intubated with an endotracheal tube. An emergency tracheotomy can establish an airway in less than 2 minutes. See Chapter 25 for a discussion of care of the patient with a tracheotomy. Patients receiving mechanical ventilation for upper airway obstruction or respiratory failure may require a tracheostomy after 7 or more days of continuous endotracheal intubation. In such cases, a tracheotomy is performed to prevent laryngeal injury and loss of tissue integrity by the endotracheal tube. OSA: Etiology and Genetic Risk. OSA: Assessment: Recognize Cues History: Patients are often unaware that they have sleep apnea. The disorder is suspected for any adult who has persistent daytime sleepiness or reports “waking up tired,” particularly if he or she also snores heavily. Ask about sensations of daytime sleepiness or falling asleep while performing tasks such as using the computer, reading, or driving. In extreme cases, patients may fall asleep while eating or any time they sit down. Ask whether the patient can recall ever being awakened by his or her own snoring and whether family members have noticed heavy snoring. Ask about the frequency of nightmares, which are associated with OSA. Also ask whether family members have ever observed the patient to have a disturbed breathing pattern while sleeping. A common pattern consists of breaths that become further apart followed by a period of no breathing (apnea), which is then followed by chest and abdominal movements that lead to gasping and snorting with partial awakening to correct the obstruction temporarily. Also ask patients who may have OSA whether they have tried to induce a deeper sleep with over-the-counter sleep aids or increased evening alcohol consumption. Many patients with OSA develop some degree of gastroesophageal reflux disease (GERD) at night. Possible causes include strong abdominal and chest movements during an apnea episode, overeating, eating or drinking close to bedtime, and lying flat while sleeping. Ask the patient whether he or she is awakened often with “heartburn,” stomach contents in the mouth, or a burning, choking sensation with coughing. Determine the frequency of such episodes. Physical Assessment/Signs and Symptoms Assess the patient’s general appearance including height and weight. Many adults with OSA are overweight, which can both cause OSA and be caused by this disorder. Examine the jaw, external neck, and chin. OSA is associated with a retracted lower jaw, smaller chin, and shorter neck. Examine the oral cavity and throat for size and shape of the pharynx, size and shape of the uvula, and tongue thickness and position, and determine whether other structures (e.g., tonsils, adenoids, pillars, soft palate) are swollen or enlarged. Chronic OSA is associated with cardiovascular changes, especially hypertension that may not respond as expected to prescribed drug therapy. Assess the patient’s blood pressure and heart rate and rhythm, as well as pulse oximetry. If the patient is being treated for hypertension, ask which drug(s) and drug dosages are used for its management. If the patient is not being treated for hypertension but blood pressure is elevated on assessment, retake the blood pressure later during the examination. Document persistent elevations. Psychosocial Assessment: Irritability and personality changes are common in adults with persistent OSA, including depression, as is a general loss of interest in social activities. Family members can be helpful in determining the presence of these psychosocial changes. Ask the patient about problems with recall, concentration, perceived energy level, and the ability to stay on task when working or studying. Some clinicians have suggested that long-term untreated OSA with significant cerebral hypoxia can lead to memory loss and dementia, although no clinical trials have been performed to explore this possibility. Analysis: Analyze Cues and Prioritize Hypotheses The primary collaborative problem for the patient with moderate-to-severe OSA is persistent poor gas exchange and hypoxia due to abnormal sleep pattern. Planning and Implementation: Generate Solutions and Take Action Improving the Duration of Restorative Sleep Planning Expected Outcomes The patient is expected to achieve a sleep pattern consistent with adequate gas exchange and longer-duration restorative sleep. Interventions Management strategies for OSA vary with the severity of the problem and the patient’s willingness to participate in the treatment process. Both nonsurgical and surgical management approaches are available to help correct the problem, depending on the cause and the severity. Far more patients are helped by nonsurgical procedures; surgical management is reserved for anatomic-related causes and severe OSA that remains resistant to nonsurgical approaches. Nonsurgical Management Collaborative management for patients with OSA focuses on reducing the obstruction and improving both the depth and the duration of restorative sleep paerns. Changes in sleeping position or weight loss may correct mild sleep apnea and improve gas exchange . More severe OSA requires additional methods to prevent obstruction. Position-fixing devices may prevent subluxation of the tongue and reduce obstruction. Use of an oral appliance, called maxillomandibular advancement, can improve airflow by supporting the lower jaw in a more forward position. These devices are especially helpful for adults who have a retracted lower jaw. Some devices also help prevent the tongue from slipping backward during sleep. These devices are bulky and some patients cannot tolerate their presence in the mouth. They may also increase the risk for loss of tissue integrity of the oral mucosa. Noninvasive positive-pressure ventilation (NPPV) via continuous positive airway pressure (CPAP) to hold open the upper airways is the most commonly used form of nonsurgical management for OSA. CPAP delivers a set positive airway pressure continuously during each cycle of inhalation and exhalation with the use of a small electric compressor and some type of delivery device such as a nasal-oral facemask, nasal mask, or nasal pillows (with or without cushioned or gel prongs). Any type of delivery mask or pillow used requires a proper and relatively tight fit to form a seal over the nose and mouth or just over the nose for successful therapy. Fig. 25.9 in Chapter 25 shows a properly fied standard nasal CPAP mask. Older CPAP setups had machines that were noisy, were not always humidified, and used larger masks, all of which contributed to reduced patient adherence with the therapy. Newer machines are very quiet and humidify the air. Many patients find the use of small masks (with or without cushioned or gel prongs) such as that shown in Fig. 26.2, rather than a full or partial mask, less intrusive and more acceptable. Smaller masks can help maintain tissue integrity . In addition, newer CPAP compressors are able to monitor many patient parameters (e.g., oxygen saturation, number and duration of apnea episodes, heart rate, air leaks, duration of correct usage, and other data) that can be sent electronically to the patient’s smart phone and/or the health care provider’s office as a way to measure therapy effectiveness. Stress to the patient that adherence with the therapy is critical in reducing all the health problem risks associated with OSA. The equipment can be expensive and much of the cost is covered by Medicare for older adults if the patient uses it consistently for at least 6 hours daily. After a p y y period of adjustment many patients who use CPAP therapy consistently sleep well and feel so much beer that adherence is less of an issue. Other patients find the equipment and routine too intrusive or unacceptable and may not even try it. Providing accurate information and patient education is therefore essential for therapy success. Many sleep apnea clinics and practices provide in-depth educational materials and patient-assistance resources to help reduce patient anxiety and promote adherence. Drug therapy for OSA has not been very successful. Sedatives to promote nighttime sleep may make OSA worse. Stimulants to help promote daytime wakefulness have many side effects and do not contribute to restorative sleep. Neither sedatives nor stimulants treat the cause of OSA. Surgical Management Surgical intervention is considered when patients are not able to tolerate CPAP or when its use does not improve OSA. Care Coordination and Transition Management Most patients who are prescribed to use CPAP for treatment of OSA are managed in the community. Usually follow-up with the primary health care provider or a sleep center is ongoing to determine treatment effectiveness. For those patients who require surgery for management of OSA, the patient is usually discharged home within 2 to 3 days. At the time of discharge pain is controlled with oral drugs and the patient should be able to swallow effectively. Self-Management Education An important issue with CPAP therapy for OSA management is appropriate maintenance of the compressor and the mask/tubular system. Keeping the system clean is critical to prevent infection and maintain tissue integrity. With humidification, fungal infections are possible. Most setups require the use of distilled water in the humidifier. The mask device or pillows must be cleaned daily using agents recommended by the specific manufacturer. Instruct the patient to not share the mask, pillows, or tubing with other people to reduce the risk for infection. Teach patients who had surgical interventions for OSA to assess the oropharynx for bleeding, swelling, or indications of infection. A small amount of blood mixed with saliva or mucus, particularly after coughing, is normal. However, new-onset bleeding, large clots, or bright red blood may indicate serious problems. Instruct the patient to notify the surgeon immediately or go to the emergency department if this should occur. Teach the patient how to examine his or her throat with a mirror twice daily and assess its internal size (often by comparing it to coin sizes). A narrowing of the throat or an inability to swallow (with or without pain), and the presence of drooling are indicators of swelling that may obstruct the airway. Instruct the patient to go to the emergency room should these occur. Pain is expected to decrease daily and swallowing should become increasingly more comfortable. Drinking cool liquids, keeping the environment humidified, gargling frequently with warm salt water, and eating soft foods can help reduce pain. Instruct the patient to report an increase in pain or increasingly greater difficulty swallowing to the surgeon. Teach the patient the indications of infection and to notify the surgeon if they occur. Infection indications include an increase in swelling, the presence of pus in any area of the oropharynx, a change in color of the mucous membrane to a “beefy red,” an increase in pain, the presence of fever, taste changes, and the presence of bad breath. Activity restriction varies depending on the exact nature of the procedure performed. Obtain a list of surgeon-directed activity restrictions and educate the patient about why each restriction has been prescribed. The most common restrictions include lifting and performing the Valsalva maneuver (holding the breath and bearing down). Psychosocial Preparation Some patients using CPAP therapy for management of OSA may have anxiety about correct use of the equipment and possible disruption of nighime routine. All patients require some period of adjustment to the therapy. Provide the patient with wrien and digital instructions for the use and care of CPAP. Also provide telephone resource numbers for health care professionals specializing in the management of OSA and for supplies needed for long-term use of CPAP. After surgery the patient may have some anxiety about whether the surgical intervention was successful and about pain and swallowing issues. Remind him or her that pain and difficulty swallowing are expected but should rapidly improve in a week. Snoring or sleep apnea may continue for a short time after surgery until all swelling is gone. Evaluation: Evaluate Outcomes Evaluate the care of the patient with obstructive sleep apnea (OSA) based on the priority patient problem. The expected outcomes are that the patient: • Does not remain hypertensive or has hypertension that can be controlled with appropriate therapy • Is adherent with prescribed nonsurgical interventions • Has fewer sleep-time apnea periods of 10 seconds or longer • Has improved gas exchange with greater duration of restorative sleep • Reports less daytime sleepiness and has more energy • Has an uneventful recovery from surgical intervention Epistaxis Pathophysiology Review Epistaxis (nosebleed) is a common problem because of the many capillaries within the nose. Nosebleeds occur as a result of loss of tissue integrity from trauma to the nasal mucosa, hypertension, blood dyscrasia (e.g., leukemia), inflammation, tumor, decreased humidity, nose blowing, nose picking, chronic cocaine use, and procedures such as nasogastric (NG) suctioning. Older adults tend to bleed most often from the posterior portion of the nose. The patient often reports that the bleeding started after sneezing or blowing the nose. Document the amount and color of the blood, and take vital signs. Ask about the number, duration, and causes of previous bleeding episodes. Review the Best Practice for Patient Safety & Quality Care box for emergency care of the patient with an anterior nosebleed. An additional intervention for use at home or in the emergency department is a nasal plug that contains an agent to promote blood clotting and expands on contact with blood to compress mucosal blood vessels. Medical attention is needed if the nosebleed does not respond to these interventions. In such cases, the affected capillaries may be cauterized with silver nitrate or electrocautery, and the nose packed. Anterior packing controls bleeding from the anterior nasal cavity. Posterior nasal bleeding is an emergency because it cannot be easily reached, and the patient may lose a lot of blood quickly. Posterior packing, epistaxis catheters (nasal pressure tubes), or gel tampons are placed through the nose within the posterior nasal region to stop the bleeding. Placement of these devices is uncomfortable; and the airway may be obstructed with reduced gas exchange if the pack slips (Schreiber, 2020). Observe the patient for respiratory distress and for tolerance of the devices. Humidity, oxygen, bedrest, and antibiotics may be prescribed. Opioid drugs may be prescribed for pain. Assess patients receiving opioids at least hourly for gag and cough reflexes. Use pulse oximetry to monitor for hypoxemia. The tubes or packing is usually removed after 1 to 3 days. For posterior bleeding that does not respond to packing or tubes, additional options include cauterizing or ligating the blood vessels or performing an embolization of the bleeding artery with interventional radiology. Potential complications of embolization include facial pain, loss of tissue integrity with necrosis of skin or nasal mucosa, facial nerve paralysis, and blindness. After the tubes or packing has been removed, teach the patient and family these interventions to use at home for comfort and safety: • Apply petroleum jelly sparingly to the nares for comfort. • Use saline nasal sprays after healing to add moisture and prevent rebleeding. • Avoid vigorous nose blowing, the use of aspirin or other NSAIDs, and strenuous activities such as heavy lifting for at least 1 month. Fractures of the nose Injury to the nose may result in loss of tissue integrity with nasal fractures that interfere with gas exchange. If the bone or cartilage is not displaced and no complications are present, treatment may not be needed. However, displacement of either the bone or cartilage can cause airway obstruction or cosmetic deformity and is a potential source of infection. Document any nasal problem, including deviation, malaligned nasal bridge, a change in nasal breathing, crackling of the skin (crepitus) on palpation, bruising, and pain. Blood or clear fluid (cerebrospinal fluid [CSF]) may drain from one or both nares as a result of a simple nasal fracture. This is rare and, if present, indicates a serious injury (e.g., skull fracture). CSF can be differentiated from normal nasal secretions because CSF contains glucose that will test positive with a dipstick test for glucose. When CSF dries on a piece of filter paper, a yellow “halo” appears as a ring at the dried edge of the fluid. Interventions: closed reduction, rhinoplasty, nasoseptoplasty Take Action The primary health care provider performs a simple closed reduction (moving the bones by palpation to realign them) of the nasal fracture using local or general anesthesia within the first 24 hours after injury. After 24 hours the fracture is more difficult to reduce because of edema and scar formation. Then reduction may be delayed for several days until edema is gone. Management focuses on pain relief and cold compresses to decrease swelling. Rhinoplasty post-op: Reduction and surgery may be needed for severe fractures or for those that do not heal properly. Rhinoplasty is a surgical reconstruction of the nose performed to repair a fractured nose and also to change the shape of the nose for improved function or appearance. Instruct the patient to stay in a semi-Fowler position and to move slowly. Suggest that he or she rest and use cool compresses on the nose, eyes, and face to help reduce swelling and bruising. After the gag reflex has returned, urge the patient to drink at least 2500 mL/day. To prevent bleeding, teach the patient to avoid forceful coughing or straining during a bowel movement, not to sniff upward or blow the nose, and not to sneeze with the mouth closed for the first few days after the packing is removed. Instruct him or her to avoid aspirin and other NSAIDs to prevent bleeding. Antibiotics may be prescribed to prevent infection. Explain that because of edema the final surgical result may require 6 to 12 months. Facial Trauma The priority action when caring for a patient with facial trauma is airway assessment for gas exchange. Signs of airway obstruction are stridor, shortness of breath, dyspnea, anxiety, restlessness, hypoxia, decreased oxygen saturation, cyanosis, and loss of consciousness. Le Fort Fracture: I—Nasoethmoid complex fracture II—Maxillary and nasoethmoid complex fracture III — Combination of I & II plus orbital-zygoma fracture; often called craniofacial disjunction Le Fort Fracture: Assessment: Recognize Cues • • • • • Airway Site of trauma Vision and eye movement Behind ears Spinal and skull trauma Le Fort Fracture: Interventions: Take Action • • Establish and maintain airway Control hemorrhage • • • Assess for extent of injury Stabilize jaw fracture (if present) Surgery may be needed Laryngeal Trauma Laryngeal trauma and damage occur with a crushing or direct-blow injury, fracture, or prolonged endotracheal intubation with loss of tissue integrity. Symptoms include difficulty breathing, inability to produce sound (aphonia), hoarseness, and subcutaneous emphysema (air present in the subcutaneous tissue). Bleeding from the airway (hemoptysis) may occur, depending on the location of the trauma. The primary health care provider performs a direct visual examination of the larynx by laryngoscopy or fiberoptic laryngoscopy to determine the extent of the injury. Management of patients with laryngeal injuries consists of assessing the effectiveness of gas exchange and monitoring vital signs (including respiratory status and pulse oximetry) every 15 to 30 minutes. Maintaining a patent airway is a priority. Apply oxygen and humidification as prescribed to maintain adequate oxygen saturation. Surgical intervention is needed for lacerations of the mucous membranes, cartilage exposure, and cord paralysis. Laryngeal repair is performed as soon as possible to prevent laryngeal stenosis and to cover any exposed cartilage. An artificial airway may be needed temporarily. Head and Neck Cancer Pathophysiology Review: Head and neck cancers are usually squamous cell carcinomas. These slowgrowing tumors are curable when diagnosed and treated at an early stage. The prognosis for those who have more advanced disease at diagnosis depends on the extent and location of the tumors. Untreated, these cancers are often fatal within 2 years of diagnosis (ACS, 2020). The cancer begins as a loss of cellular regulation when the mucosa is chronically irritated and becomes tougher and thicker. Eventually genes controlling cell growth are damaged, allowing excessive growth of these abnormal and malignant cells. Initial lesions first appear as white, patchy lesions (leukoplakia) or red, velvety patches (erythroplakia). Head and neck cancer first spreads (metastasizes) into local lymph nodes, muscle, and bone. Later spread is systemic to distant sites, usually to the lungs or liver. Head and Neck Cancer risk factors: Tobacco and alcohol use Voice abuse Chronic laryngitis Exposure to chemicals or dust Poor oral hygiene Long-term GERD Oral infection with HPV Assessment: Recognize Cues Ask about tobacco and alcohol use, history of acute or chronic laryngitis or pharyngitis, oral sores, swallowing difficulty, and lumps in the neck. Calculate the patient’s pack-years of smoking history (see Chapter 24). Ask about alcohol intake (how many drinks per day and for how many years). Also ask about oral exposure to HPV (Schiech, 2016), which has been recognized as an increasing cause of head and neck cancer. Table 26.1 lists the warning signs of head and neck cancer. With laryngeal cancer, painless hoarseness may occur because of tumor size and an inability of the vocal cords to come together for normal speech. Any adult who has a history of hoarseness, mouth sores, or a lump in the neck for 3 to 4 weeks should be evaluated for laryngeal cancer. Imaging assessment ◦ X-rays, CT, MRI, SPECT, PET-CT Interventions Radiation therapy Chemotherapy Biotherapy Surgical intervention Post-op care: Head and neck surgery often lasts 8 hours or longer, and the patient spends the immediate period after surgery in an ICU. Monitor airway patency, vital signs, hemodynamic status, and comfort level. Take vital signs and monitor for hemorrhage and other general complication of anesthesia and surgery hourly for the first 24 hours and then according to agency policy until the patient is stable. Complications after surgery include airway obstruction, hemorrhage, wound breakdown, and tumor recurrence. The first priorities after head and neck surgery are airway maintenance and ensuring gas exchange. Maintaining the Airway and Gas Exchange Immediately after surgery, the patient may need mechanical ventilation. During weaning, the patient usually uses a tracheostomy collar (over the artificial airway or open stoma) with oxygen and humidity to help move mucus secretions. Secretions may remain blood-tinged for 1 to 2 days. Use Standard Precautions, and report any increase in bleeding to the surgeon. A laryngectomy tube is used for patients who have undergone a total laryngectomy and need an appliance to prevent scar tissue shrinkage of the skin-tracheal border. This tube is similar to a tracheostomy tube but is shorter and wider with a larger lumen. Care is similar to tracheostomy tube care (see Chapter 25) except that the patient can change the laryngectomy tube daily or as needed. A laryngectomy buon is similar to a laryngectomy tube but is softer, has a single lumen, and is very short. Provide alternative communication techniques because the patient cannot speak. Managing the Wound Stoma care after a total laryngectomy is a combination of wound care and airway care. Inspect the stoma and clean the suture line with sterile saline (or a prescribed solution) to prevent secretions from forming crusts and obstructing the airway. Perform suture line care every 1 to 2 hours during the first few days after surgery and then every 4 hours. The mucosa of the stoma and trachea should be bright pink and shiny and without crusts, similar to the appearance of the oral mucosa. Tissue “flaps” may be used to close the wound and improve appearance. Flaps are skin, subcutaneous tissue, and sometimes muscle, taken from other body areas and used for reconstruction after head and neck resection. The first 24 hours after surgery are critical. Evaluate all grafts and flaps hourly for the first 72 hours. Monitor capillary refill, color, drainage, and Doppler activity of the major blood vessel to the area. Report changes to the surgeon immediately because surgical intervention may be needed. Position the patient so the surgical flaps are not dependent. Wound breakdown with loss of tissue integrity is a common complication in patients after head and neck surgery, especially if radiation therapy occurred before surgery. Manage wound breakdown with packing and local care as prescribed to keep the wound clean and stimulate the growth of healthy granulation tissue. Wounds may be extensive, and the carotid artery may be exposed, which increases the risk for rupture and hemorrhage. Managing Pain Pain after surgery has many causes and should be managed while still allowing the patient to be able to participate in care. Morphine often is given IV by a patient-controlled analgesia (PCA) pump for the first 1 to 2 days after surgery. As the patient progresses, liquid opioid analgesics can be given by feeding tube. Oral drugs for pain and discomfort are started only after the patient can tolerate oral intake. Maintaining Nutrition All patients are at risk for malnutrition during treatment for head and neck cancer. A nasogastric (NG), gastrostomy, or jejunostomy tube is placed during surgery for nutrition support while the head and neck heal and may remain in place for 7 to 10 days. It is removed when the patient is able to swallow safely. Aspiration cannot occur after a total laryngectomy because the airway is completely separated from the esophagus. Promoting Communication The patient’s voice quality and speech are altered after surgery. This problem has enormous effects on the patient’s social interactions, continued employment, and quality of life. In collaboration with an SLP, work with the patient and family toward developing an acceptable communication method during the inpatient period. Speech production varies with patient practice, amount of tissue removed, and radiation effects but can be very understandable. The speech rehabilitation plan for patients who have a total laryngectomy at first consists of writing, using a picture board, smart phone, or computer. The patient then uses an artificial larynx and may eventually learn esophageal speech. He or she needs encouragement and support from the SLP, hospital team, and family while relearning to speak. Having a laryngectomee (an adult who has had a laryngectomy) from one of the local self-help organizations, such as the ACS Visitor Program or the International Association of Laryngectomees, visit the patient and family is often beneficial. Preventing Aspiration The surgical changes in the upper respiratory tract and altered swallowing mechanisms increase the patient’s risk for aspiration. Aspiration can result in pneumonia, weight loss, and prolonged hospitalization. A nasogastric (NG) feeding tube increases the risk for aspiration because it keeps the lower esophageal sphincter partially open. Most patients who need enteral feeding supplementation have a percutaneous endoscopic gastrostomy (PEG) tube placed rather than an NG tube. See Chapter 55 for care of patients receiving enteral nutrition by NG or PEG tube. Swallowing can be a problem for the patient who has a tracheostomy tube. It can be normal if the cranial nerves and anatomic structures are intact. In a normal swallow, the larynx rises and moves forward to protect itself from the passing stream of food and saliva. The tracheostomy tube may fix the larynx in place Supporting Self-Esteem The patient with head and neck cancer usually has a change in selfconcept and self-image resulting from issues such as the presence of a stoma or artificial airway, speech changes, and a change in the method of eating. Psychosocial issues may include guilt, regret, and uncertainty. He or she may not be able to speak at all or may have permanent speech deficits. Help the patient set realistic goals, starting with involvement in self-care. Reinforce the alternative communication methods suggested by the SLP so the patient can communicate in the hospital and after discharge. After surgery, the patient may feel socially isolated because of the change in voice and facial appearance. Loose-fiing, high-collar shirts or sweaters, scarves, and jewelry can be worn to cover the laryngectomy stoma, tracheostomy tube, and other changes related to surgery. Cosmetics may aid in covering any facial or neck disfigurement. Managing Pain Pain after surgery has many causes and should be managed while still allowing the patient to be able to participate in care. Morphine often is given IV by a patient-controlled analgesia (PCA) pump for the first 1 to 2 days after surgery. As the patient progresses, liquid opioid analgesics can be given by feeding tube. Oral drugs for pain and discomfort are started only after the patient can tolerate oral intake. Maintaining Nutrition All patients are at risk for malnutrition during treatment for head and neck cancer. A nasogastric (NG), gastrostomy, or jejunostomy tube is placed during surgery for nutrition support while the head and neck heal and may remain in place for 7 to 10 days. It is removed when the patient is able to swallow safely. Aspiration cannot occur after a total laryngectomy because the airway is completely separated from the esophagus. Promoting Communication The patient’s voice quality and speech are altered after surgery. This problem has enormous effects on the patient’s social interactions, continued employment, and quality of life. In collaboration with an SLP, work with the patient and family toward developing an acceptable communication method during the inpatient period. Speech production varies with patient practice, amount of tissue removed, and radiation effects but can be very understandable. The speech rehabilitation plan for patients who have a total laryngectomy at first consists of writing, using a picture board, smart phone, or computer. The patient then uses an artificial larynx and may eventually learn esophageal speech. He or she needs encouragement and support from the SLP, hospital team, and family while relearning to speak. Having a laryngectomee (an adult who has had a laryngectomy) from one of the local self-help organizations, such as the ACS Visitor Program or the International Association of Laryngectomees, visit the patient and family is often beneficial. Preventing Aspiration The surgical changes in the upper respiratory tract and altered swallowing mechanisms increase the patient’s risk for aspiration. Aspiration can result in pneumonia, weight loss, and prolonged hospitalization. A nasogastric (NG) feeding tube increases the risk for aspiration because it keeps the lower esophageal sphincter partially open. Most patients who need enteral feeding supplementation have a percutaneous endoscopic gastrostomy (PEG) tube placed rather than an NG tube. See Chapter 55 for care of patients receiving enteral nutrition by NG or PEG tube. Swallowing can be a problem for the patient who has a tracheostomy tube. It can be normal if the cranial nerves and anatomic structures are intact. In a normal swallow, the larynx rises and moves forward to protect itself from the passing stream of food and saliva. The tracheostomy tube may fix the larynx in place, Home Care Management Extensive home care preparation is needed after a laryngectomy for cancer. The convalescence period is long, and airway management is complicated. For the patient with severe respiratory problems, home changes to allow for one-floor living may be needed. Increased humidity is needed. A humidifier add-on to a forced-air furnace can be obtained, or a room humidifier or vaporizer may be used. Be sure to stress that meticulous cleaning of these items is needed to prevent spread of mold or other sources of infection. A home care nurse is often an important resource for the patient and family. This nurse assesses the patient and home situation for problems in self-care, complications, adjustment, and adherence to the medical regimen. Self-Management Education Teach the patient and family how to care for the stoma or tracheostomy or laryngectomy tube, depending on the type of surgery performed, using the actions listed in the Patient and Family Education: Preparing for Self Management: Home Laryngectomy Care box. Stoma care teaching is focused on protection, which is needed as a result of the anatomic changes resulting from surgery. Instruct the patient to use a shower shield over the tube or stoma when bathing to prevent water from entering the airway. Suggest that the patient wear a protective cover or stoma guard to protect the stoma during the day. Communication involves having the patient continue the selected communication method that began in the hospital. Instruct him or her to wear a medical alert (MedicAlert) bracelet and carry a special identification card. For patients with a laryngectomy, this card is available from the local chapters of the International Association of Laryngectomees. The card instructs the reader about providing an emergency airway or resuscitating someone who has a stoma Psychosocial Preparation The many changes resulting from a laryngectomy influence physical, social, and emotional functioning for both the patient and his or her significant other (Sterba et al., 2016). The patient with a permanent stoma, tracheostomy tube, NG or PEG tube, and wounds has an altered body image. Stress the importance of returning to as normal a lifestyle as possible. Most patients can resume many of their usual activities within 4 to 6 weeks after surgery. The patient with a total laryngectomy cannot produce sounds during laughing and crying. Mucus secretions may appear unexpectedly when these emotions arise or when coughing or sneezing occurs. The mucus can be embarrassing, and the patient needs to be prepared to cover the stoma with a handkerchief or gauze. The patient who has undergone composite resections has difficulty with speech and swallowing. He or she may need to deal with tracheostomy and feeding tubes in public places. 27: Concepts of Care for Patients With Noninfectious Lower Respiratory Problems KEY TERMS asthma: A chronic disease in which acute reversible airway obstruction occurs intermiently, reducing airflow. chronic bronchitis: An inflammation of the bronchi and bronchioles caused by exposure to irritants, especially cig smoke. chronic obstructive pulmonary disease (COPD): A collection of lower airway disorders that interfere with airflow and gas exchange. control therapy drugs: Asthma drugs used daily to reduce airway sensitivity (responsiveness) to prevent asthma attacks from occurring and to maintain gas exchange. cor pulmonale: Right-sided heart failure caused by pulmonary disease occurring with bronchitis or emphysema. cystic fibrosis (CF): An autosomal recessive genetic disease that affects many organs with most impairment occurring to pancreatic and/or lung function. dyspnea: Perceived shortness of breath. emphysema: A destructive problem of lung elastic tissue that reduces its ability to recoil after stretching, leading to hyperinflation of the lung. hypercapnia: Higher than normal blood carbon dioxide levels. Also known as hypercarbia. hypoxemia: Low blood oxygen levels. lobectomy Removal of a lobe of the lung. orthopnea: Breathlessness that is worse in a supine position. pneumonectomy Surgical removal of an entire lung. pulmonary artery hypertension (PAH) A condition in which pulmonary vessels and often other lung tissues undergo growth changes that greatly increase pressure in the lung circulatory system for unknown reasons (also known as idiopathic pulmonary artery hypertension). reliever drugs: Asthma drugs used to stop an asthma attack once it has started. Also known as rescue drugs. The lower respiratory tract is the tubular system of the trachea (below the larynx), two mainstem bronchi, five secondary bronchi, thousands of branching bronchi and bronchioles, and the alveolar ducts, which connect to the final portions of the tract, the alveoli. Air must flow through the entire tubular system for needed oxygen to reach the alveolar ducts and alveoli where primary gas exchange occurs. When the function of any of these structures is reduced, both gas exchange and systemic perfusion are impaired. Asthma Pathophysiology Review One of the most common lower respiratory disorders that reduces gas exchange is asthma, which can lead to severe lower airway obstruction and death. Asthma is a chronic disease in which reversible acute airway obstruction occurs intermittently, reducing airflow (Fig. 27.1). Airway obstruction occurs by both inflammation and airway tissue sensitivity (hyperresponsiveness) with bronchoconstriction. Inflammation obstructs the airway lumens (i.e., the hollow insides) (Fig. 27.2). Airway hyperresponsiveness and constriction of bronchial smooth muscle narrow the tubular structure of the airways. Airway inflammation and sensitivity can trigger bronchiolar constriction, and many adults with asthma have both problems (McCance et al., 2019). More than 3300 deaths from acute asthma occur in the United States each year (Centers for Disease Control and Prevention [CDC], 2019a). Etiology and Genetic Risk: Although asthma may be classified into types based on what triggers the attacks, the effect on gas exchange is the same. Inflammation of the mucous membranes lining the airways is a key event in triggering an asthma attack. It occurs in response to the presence of specific allergens; general irritants such as cold air, dry air, or fine airborne particles; microorganisms; and aspirin and other NSAIDs. Incidence: Asthma can occur at any age. About half of adults with asthma also had the disease in childhood. Asthma affects over 22 million adults in the United States and Canada (19.9 million in the United States and 2.6 million in Canada) (CDC, 2018b; Statistics Canada, 2017 ). It is more common in urban settings than rural settings. Assessment: Recognize Cues History The patient with asthma usually has a pattern of intermittent episodes of dyspnea (perceived shortness of breath), chest tightness, coughing, wheezing, and increased mucus production. Ask whether the symptoms occur continuously, seasonally, in association with specific activities or exposures, at work, or more frequently at night. Some patients have symptoms for 4 to 8 weeks after a cold or other upper respiratory infection. The patient with atopic (allergic) asthma also may have other allergic problems. Ask whether any family members have asthma or respiratory problems. Ask about current or previous smoking habits. If the patient smokes, use this opportunity to teach him or her about smoking cessation (see Chapter 24). Wheezing in nonsmokers is important in the diagnosis of asthma. Physical Assessment/Signs and Symptoms The patient with mild to moderate asthma may have no symptoms between asthma attacks. During an acute episode, common symptoms are an audible wheeze and increased respiratory rate. At first the wheeze is louder on exhalation. When inflammation occurs with asthma, coughing may increase. The patient may use accessory muscles to help breathe during an aack. Observe for muscle retraction at the sternum and the suprasternal notch and between the ribs. The patient with long-standing, severe asthma may have a “barrel chest,” caused by air trapping (Fig. 27.3). The anteroposterior (AP) diameter (diameter between the front and the back of the chest) increases with air trapping, giving the chest a rounded rather than an oval shape. The normal chest is about 1.5 times as wide as it is deep. In severe, chronic asthma, the AP diameter may equal or exceed the lateral diameter (Jarvis, 2020). Compare the chest AP diameter with the lateral diameter. Chronic air trapping also flaens the diaphragm and increases the space between the ribs. Along with an audible wheeze, the breathing cycle is longer, with prolonged exhalation, and requires more effort. The patient may be unable to speak more than a few words between breaths. Hypoxia occurs with severe aacks. Pulse oximetry shows hypoxemia (poor blood oxygen levels). Examine the oral mucosa and nail beds for cyanosis. Other indicators of hypoxemia include changes in the level of cognition or consciousness and tachycardia Laboratory Assessment Laboratory tests can determine asthma type and the degree of breathing impairment. Arterial blood gas (ABG) levels show the effectiveness of gas exchange (see Chapter 14 for discussion of ABGs). The arterial oxygen level (PaO 2 ) may decrease during an asthma aack. Early in the aack, the arterial carbon dioxide level (PaCO 2 ) may be decreased as the patient increases the breathing rate and depth. Later in an asthma episode, PaCO 2 rises, as does the end-tidal carbon dioxide level, indicating carbon dioxide retention. Allergic asthma often occurs with elevated serum eosinophil counts and immunoglobulin E (IgE) levels. The sputum may contain eosinophils and mucus plugs with shed epithelial cells (Curschmann spirals). Pulmonary Function Tests The most accurate tests for measuring airflow in asthma are the pulmonary function tests (PFTs) using spirometry. Baseline PFTs are obtained for all patients diagnosed with asthma. The most important PFTs for a patient with asthma are the forced vital capacity (FVC), the forced expiratory volume in the first second (FEV1 ), and the peak expiratory flow (PEF), sometimes called peak expiratory rate flow (PERF). Definitions of PFTs are listed in Chapter 24. A decrease in either the FEV1 or the PEF (PERF) of 15% to 20% below the expected value for age, gender, and size is common for the patient with asthma (Durham et al., 2017). Asthma is diagnosed when these values increase by 12% or more after treatment with bronchodilators. Airway responsiveness is tested by measuring the PEF and FEV1 before and after the patient inhales the drug methacholine, which induces bronchospasm in susceptible adults Interventions: Take Action The purposes of asthma therapy are to control and prevent episodes, improve airflow and gas exchange , and relieve symptoms. Asthma is best controlled when the patient is an active partner in the management plan. Priority nursing actions focus on patient education about using his or her personal asthma action plan, which includes drug therapy and lifestyle management strategies to help him or her understand the disease and its management. Self-Management Education Asthma often has intermittent overt symptoms. With guided self-care, patients can co-manage this disease, increasing symptom-free periods and decreasing the number and severity of attacks. Good management decreases hospital admissions and increases participation in patient chosen work and leisure activities. Drug Therapy Control therapy drugs are used to reduce airway sensitivity (responsiveness) to prevent asthma attacks from occurring and maintain gas exchange . They are used every day, regardless of symptoms. Reliever drugs (also called rescue drugs) are used to actually stop an attack once it has started. Some patients may need drug therapy only during an asthma episode. For others, daily drugs are needed to keep asthma episodic rather than a more frequent problem. Therapy involves the use of bronchodilators and various drug types to reduce inflammation. Some drugs reduce the asthma response, and other drugs actually prevent it. Combination drugs are two or more agents from different classes combined together for beer response. With step therapy, drug therapy is prescribed at different levels, starting with step 1 and progressing up (stepping up), until acceptable control is achieved. When the patient achieves control and maintains it for 3 months, the respiratory health care provider adjusts the drug regimen down a step (stepping down) at a time to reach and maintain a goal of good control at the lowest possible drug dosages. • • • Drug therapy • Control therapy drugs (used daily) • Reliever drugs (used to stop an attack) • Bronchodilators • Anti-inflammatory agents Exercise and activity Oxygen therapy Status Asthmaticus Status asthmaticus is a severe, life-threatening acute episode of airway obstruction that intensifies once it begins and often does not respond to usual therapy. The patient arrives in the emergency department with extremely labored breathing and wheezing. Use of accessory muscles for breathing and distention of neck veins are observed. If the condition is not reversed, the patient may develop pneumothorax and cardiac or respiratory arrest. IV fluids, potent systemic bronchodilators, steroids, epinephrine, and oxygen are given immediately to reverse the condition. Magnesium sulfate also may be used, although this practice is controversial. Prepare for emergency intubation. Gas Exchange Concept Exemplar: Chronic Obstructive Pulmonary Disease Pathophysiology Review Chronic obstructive pulmonary disease (COPD) is a collection of lower airway disorders that interfere with airflow and gas exchange. These disorders include emphysema and chronic bronchitis. Etiology and Genetic Risk Cigarette smoking is the greatest risk factor for COPD. The patient with a 20–pack-year history or longer often has early-stage COPD with changes in pulmonary function tests (PFTs). The inhaled smoke triggers the release of excessive proteases in the lungs. These enzymes break down elastin, the major component of alveoli. By impairing the action of cilia, smoking also inhibits the cilia from clearing the bronchi of mucus, cellular debris, and fluid. Alpha 1 -antitrypsin deficiency is a less common but important risk factor for COPD, although it is often underrecognized (Southard et al., 2020). Complications COPD affects gas exchange and the oxygenation of all tissues. Complications include hypoxemia, acidosis, respiratory infection, cardiac failure, dysrhythmias, and respiratory failure. Hypoxemia and acidosis occur because the patient with COPD has reduced gas exchange, leading to decreased oxygenation and increased carbon dioxide levels. These problems reduce cellular function. Respiratory infection risk increases because of the increased mucus and poor gas exchange . Bacterial infections are common and make COPD symptoms worse by increasing inflammation and mucus production and inducing more bronchospasm. Airflow becomes even more limited, the work of breathing increases, and dyspnea results. Cardiac failure, especially cor pulmonale (right-sided heart failure caused by pulmonary disease), occurs with bronchitis or emphysema. Cardiac dysrhythmias are common in patients with COPD Health Promotion and Maintenance The incidence and severity of COPD would be greatly reduced by smoking cessation. Urge all adults who smoke to quit smoking or vaping and to avoid particulate maer exposure using the I-PREPARE model as described in Chapter 24. Assessment: Recognize Cues History: Ask about risk factors such as age, gender, and occupational history. COPD is seen more often in older men. Some types of emphysema occur in families, especially those with alpha1 -antitrypsin (AAT) deficiency (Southard et al., 2020). Obtain a thorough smoking history because tobacco use is a major risk factor. Ask about the length of time the patient has smoked and the number of packs smoked daily. Use these data to determine the pack-year smoking history. Also ask about the use of electronic cigarettes or vaping, including the type of product inhaled and the duration of the habit. Ask the patient to describe the breathing problems and assess whether he or she has any difficulty breathing while talking. Does he or she speak in complete sentences, or is it necessary to take a breath between every one or two words? Ask about the presence, duration, or worsening of wheezing, coughing, and shortness of breath. Determine which activities trigger these problems. Assess any cough, and ask whether sputum is clear or colored and how much is produced each day. Ask about the time of day when sputum production is greatest. Smokers often have a productive cough when they get up in the morning; nonsmokers generally do not. Ask the patient to compare the activity level and shortness of breath now with those of a month ago and a year ago. Ask about any difficulty with eating and sleeping. Many patients sleep in a semisiing position because breathlessness is worse when lying down (orthopnea). Ask about any difficulty with ADLs or sexual activity. Document this assessment to personalize the intervention plan. Weigh the patient and compare this weight with previous weights. Unplanned weight loss is likely when COPD severity increases, because the work of breathing increases metabolic needs. Dyspnea and mucus production often result in poor food intake and inadequate nutrition. Ask the patient to recall a typical day’s meals and fluid intake. When heart failure is present with COPD, general edema with weight gain may occur. Physical Assessment/Signs and Symptoms General appearance can provide clues about respiratory status and energy level. Observe weight in proportion to height, posture, mobility, muscle mass, and overall hygiene. The patient with increasingly severe COPD is thin, with loss of muscle mass in the extremities, although the neck muscles may be enlarged. He or she tends to be slow moving and slightly stooped. The patient often sits in a forwardbending posture with the arms held forward, a position known as the orthopneic or tripod position (Fig. 27.7). When dyspnea becomes severe, activity intolerance may be so great that bathing and general grooming are neglected. Respiratory changes that occur as a result of obstruction include changes in chest size, and fatigue. Inspect the chest and assess the breathing rate and pattern. The patient with respiratory muscle fatigue breathes with rapid, shallow respirations and may have an abnormal breathing pattern in which the abdominal wall is sucked in during inspiration or may use accessory muscles in the abdomen or neck. Psychosocial Assessment COPD affects all aspects of a patient’s life. He or she may be isolated because dyspnea causes fatigue or because of embarrassment from coughing and excessive sputum production. Ask the patient about interests and hobbies to assess whether socialization has decreased or whether hobbies cause exposure to irritants. Ask about home conditions for exposure to smoke or crowded living conditions that promote transmission of respiratory infections. Economic status may be affected by the disease through changes in income and health insurance coverage. Drugs delivered by inhalers are expensive, and many patients with limited incomes may use them only during exacerbations and not as prescribed on a scheduled basis. Anxiety and fear from feelings of breathlessness may reduce the patient’s ability to participate in a full life. Work, family, social, and sexual roles can be affected. Encourage the patient and family to express their feelings about disease progression and the limitations on lifestyle. Assess their use of support groups and community services. Laboratory Assessment Arterial blood gas (ABG) values identify abnormal gas exchange , oxygenation, ventilation, and acid-base status. Compare repeated ABG values to assess changes in respiratory status. Once baseline ABG values are obtained, pulse oximetry can gauge treatment response. A WBC count helps confirm the presence of infection. Imaging Assessment Chest x-rays are used to rule out other lung diseases and to check the progress of patients with respiratory infections or chronic disease. With advanced emphysema, chest x-rays show hyperinflation with widely spaced ribs and a flaened diaphragm. Other Diagnostic Assessments COPD is classified from mild to very severe on the basis of symptoms and pulmonary function test (PFT) changes (Table 27.2; see Table 24.6). Airflow rates and lung volume measurements help distinguish airway disease (obstructive diseases) from interstitial lung disease (restrictive diseases). PFTs determine lung volumes, flow volume curves, and diffusion capacity. Analysis: Analyze Cues and Prioritize Hypotheses The priority collaborative problems for patients with chronic obstructive pulmonary disease (COPD) include: 1. Decreased gas exchange due to alveolar-capillary membrane changes, reduced airway size, ventilatory muscle fatigue, excessive mucus production, airway obstruction, diaphragm flattening, fatigue, and decreased energy 2. Weight loss due to dyspnea, excessive secretions, anorexia, and fatigue 3. Anxiety due to a change in health status and situational crisis 4. Decreased endurance due to fatigue, dyspnea, and an imbalance between oxygen supply and demand 5. Potential for pneumonia or other respiratory infections Planning and Implementation: Generate Solutions and Take Action Improving Gas Exchange and Reducing Carbon Dioxide Retention Planning: Expected Outcomes The patient with COPD is expected to attain and maintain gas exchange at his or her usual baseline level. Interventions Most patients with COPD use nonsurgical management to improve or maintain gas exchange . Surgical management requires that the patient meet strict criteria. Nursing care is most successful with helping the patient become a partner in COPD management by participating in all therapies to improve gas exchange . Thus priority nursing management for patients with COPD focuses on ensuring consistent use of prescribed drug therapy and on airway maintenance, monitoring, breathing techniques, positioning, effective coughing, oxygen therapy, exercise conditioning, suctioning, and hydration. Before any intervention, assess the breathing rate, rhythm, depth, and use of accessory muscles. The accessory muscles are less efficient than the diaphragm, and the work of breathing increases. Determine whether any factors are contributing to the increased work of breathing, such as respiratory infection. Airway maintenance is the most important focus of interventions to improve gas exchange Drug Therapy Drug therapy is recommended at all levels of disease to delay progression and promote continued activity tolerance (GOLD, 2019; O’Dell et al., 2018). Drugs used to manage COPD are the same drugs as for asthma and include beta-adrenergic agents, cholinergic antagonists, xanthines, corticosteroids, and cromones (see the Common Examples of Drug Therapy: Asthma Prevention and Treatment box in the Asthma section). The focus is on long-term control therapy with longer-acting drugs, such as arformoterol, indacaterol, tiotropium, aclidinium bromide, olodaterol, and the combination drugs, such as fluticasone/vilanterol (e.g., BREO ELLIPTA), olodaterol/tiotropium, vilanterol/umeclidinium (e.g., ANORO ELLIPTA), and a newer triple combination of fluticasone/umeclidinium/vilanterol (e.g., TRELEGY ELLIPTA). Just as for asthma management, a key issue for successful drug therapy for COPD management is the correct technique for inhaler use. Have the patient demonstrate or fully describe exactly how he or she uses the inhaler(s) at every new outpatient interaction. Reinforce correct technique use and apply a variety of interventions that can help the patient acquire the knowledge and skills needed to use the inhaler correctly (see the Systems Thinking and Quality Improvement: Reducing Critical Errors in Patient Inhaler Use box). The patient with COPD is more likely to be taking systemic agents in addition to inhaled drugs. Another drug class for COPD is the mucolytics, which thin the thick secretions, making them easier to cough up and expel. Nebulizer treatments with normal saline or a mucolytic agent such as acetylcysteine or dornase alfa and normal saline help thin secretions. Guaifenesin is a systemic mucolytic that is taken orally. A combination of guaifenesin and dextromethorphan also raises the cough threshold. Breathing Techniques Diaphragmatic or abdominal and pursed-lip breathing may be helpful for managing dyspneic episodes. Teach the patient to use these techniques, shown in the Patient and Family Education: Preparing for SelfManagement: Breathing Exercises box, during all activities to reduce the amount of stale air in the lungs and manage dyspnea. Teach these techniques when the patient has less dyspnea. Preventing Weight Loss Planning: Expected Outcomes The patient with COPD is expected to achieve and maintain a body weight within 10% of ideal. Interventions: The patient with COPD often has nausea, early satiety (feeling too “full” to eat), poor appetite, and meal-related dyspnea. The work of breathing raises calorie and protein needs, which can lead to protein-calorie malnutrition. Malnourished patients lose muscle mass and strength, lung elasticity, and alveolar-capillary surface area, all of which reduce gas exchange. Identify patients at risk for or who have this complication and collaborate with a registered dietitian nutritionist (RDN) to perform a nutrition assessment. Monitor weight and other indicators of nutrition, such as serum prealbumin levels. Dyspnea management is needed because shortness of breath interferes with eating. Teach the patient to plan the biggest meal of the day for the time when he or she is most hungry and well rested. Four to six small meals a day may be preferred to three larger ones. Remind patients to use pursed-lip and abdominal breathing and to use the prescribed bronchodilator 30 minutes before the meal to reduce bronchospasm. Food selection can help prevent weight loss. Abdominal bloating and a feeling of fullness often prevent the patient from eating a complete meal. Collaborate with the RDN to teach about foods that are easy to chew and not gas forming. Advise the patient to avoid dry foods that stimulate coughing and caffeine-containing drinks that increase urine output and may lead to dehydration. Urge the patient to eat high-calorie, high-protein foods. Some supplements are formulated for patients with COPD and provide nutrition with reduced carbon dioxide production. If early satiety is a problem, advise him or her to avoid drinking fluids before and during the meal and to eat smaller, more frequent meals Minimizing Anxiety Planning: Expected Outcomes The patient with COPD is expected to have decreased anxiety. Interventions Patients with COPD become anxious during acute dyspneic episodes, especially when excessive secretions are present. Anxiety also may cause dyspnea. Help the patient understand that anxiety can increase dyspnea and have a plan for dealing with anxiety. Together with the patient, develop a wrien plan that states exactly what he or she should do if symptoms flare. Having a plan provides confidence and control in knowing what to do, which often helps reduce anxiety. Stress the use of pursed-lip and diaphragmatic breathing techniques during periods of anxiety or panic. Family, friends, and support groups can be helpful. Recommend professional counseling, if needed, as a positive suggestion. Stress that talking with a counselor can help identify techniques to maintain control over dyspnea and panic. Explore other approaches to help the patient manage dyspneic episodes and panic aacks, such as progressive relaxation, hypnosis therapy, and biofeedback. For some patients, antianxiety drug therapy may be needed for severe anxiety. Improving Endurance Planning: Expected Outcomes The patient with COPD is expected to increase activity to a level acceptable to him or her. Interventions The patient with COPD often has chronic fatigue. During acute exacerbations, he or she may need extensive help with the ADLs of eating, bathing, and grooming. As the acute problem resolves, encourage the patient to pace activities and perform as much self-care as possible. Teach him or her to not rush through morning activities because rushing increases dyspnea, fatigue, and hypoxemia. As activity gradually increases, assess the patient’s response by noting skin color changes, pulse rate and regularity, oxygen saturation, and work of breathing. Suggest the use of oxygen during periods of high energy use such as bathing or walking. Energy conservation is the planning and pacing of activities for best tolerance and minimum discomfort. Ask the patient to describe a typical daily schedule. Help him or her divide each activity into its smaller parts to determine whether that task can be performed in a different way or at a different time. Teach about planning and pacing daily activities with rest periods between activities. Help the patient develop a chart outlining the day’s activities and planned rest periods. Encourage the patient to avoid working with the arms raised. Activities involving the arms decrease exercise tolerance because the accessory muscles are used to stabilize the arms and shoulders rather than to assist breathing. Many activities involving the arms can be done sttiing at a table leaning on the elbows. Teach the patient to adjust work heights to reduce back strain and fatigue. Remind him or her to keep arm motions smooth and flowing to prevent jerky motions that waste energy. Work with the occupational therapist to teach about the use of adaptive tools for housework, such as long-handled dustpans, sponges, and dusters, to reduce bending and reaching. Suggest organizing work spaces so that items used most often are within easy reach. Measures such as dividing laundry or groceries into small parcels that can be handled easily, using disposable plates to save washing time, and leing dishes dry in the rack also conserve energy. Teach the patient to not talk when engaged in other activities that require energy, such as walking. In addition, teach him or her to avoid breath holding while performing any activity Preventing Respiratory Infection Planning: Expected Outcomes The patient with COPD is expected to avoid serious respiratory infection. Interventions Pneumonia is a common complication ofCOPD, especially among older adults. Patients who have excessive secretions are at increased risk for respiratory tract infections. Teach patients to avoid crowds, and stress the importance of receiving a pneumonia vaccination and a yearly influenza vaccine Home Care Management Most patients with COPD are managed in the ambulatory care setting and cared for at home. When pneumonia or a severe exacerbation develops, the patient often returns home after hospitalization. For those with advanced disease, 24-hour care may be needed for ADLs and monitoring. If home care is not possible, placement in a long-term care seing may be needed. Patients with hypoxemia may use oxygen at home either as needed or continually. Continuous, long-term oxygen therapy can reverse tissue hypoxia and improve cognition and well-being. For more information on home oxygen therapy, see Chapter 25. Collaborate with the case manager to obtain the equipment needed for care at home. Patient needs may include oxygen therapy, a hospital-type bed, a nebulizer, a tub transfer bench or shower chair, and scheduled visits from a home care nurse for monitoring and evaluation. The patient with COPD faces a lifelong disease with remissions and exacerbations. Explain to the patient and family that he or she may have periods of anxiety, depression, and ineffective coping. Self-Management Education Patients with COPD need to know as much about the disease as possible so that they can beer manage it and themselves. Patients and families should be able to discuss drug therapy, correct use of inhalers, signs of infection, avoidance of respiratory irritants, the nutrition therapy regimen, and activity progression. Instruct them to identify and avoid stressors that can worsen the disease. Reinforce the techniques of pursed-lip breathing, diaphragmatic breathing, positioning, relaxation therapy, energy conservation, and coughing and deep breathing. Teaching about all of the needed topics may require coordination with the home care or clinic staff. Evaluation: Evaluate Outcomes Evaluate the care of the patient with COPD based on the identified priority patient problems. The expected outcomes of care are that the patient will: Attain and maintain gas exchange at a level within his or her chronic baseline values • Cough and clear secretions effectively • Maintain a respiratory rate and rhythm appropriate to his or her activity level • Achieve an effective breathing pattern that decreases the work of breathing • Maintain a patent airway • Achieve and maintain a body weight within 10% of his or her ideal weight • Have decreased anxiety • Increase activity to a level acceptable to him or her • Perform ADLs with no or minimal assistance • Avoid serious respiratory infections • Use prevention activities such as pneumonia and influenza vaccination and crowd avoidance Cystic Fibrosis Pathophysiology Review Cystic fibrosis (CF) is an autosomal recessive genetic disease that affects many organs with most impairment occurring to pancreatic and/or lung function. Although CF is present from birth and usually is first seen in early childhood, almost half of patients with CF in the United States are adults (Cystic Fibrosis Foundation [CFF], 2019 ; Lomas & Tran, 2020). The underlying problem of CF is blocked chloride transport in the cell membranes. Poor chloride transport causes the formation of mucus that has little water content and is thick. The thick, sticky mucus causes problems in the lungs, pancreas, liver, salivary glands, and testes. The mucus plugs up the airways in the lungs and the glandular tissues in nonpulmonary organs, causing atrophy and organ dysfunction. Nonpulmonary problems include pancreatic insufficiency, malnutrition, intestinal obstruction, poor growth, male sterility, and cirrhosis of the liver. Additional problems of CF in young adults include osteoporosis and diabetes mellitus. Respiratory failure is the main cause of death. Improved management has increased life expectancy, even among those with severe disease, to about 47 years (CFF, 2019; Loas & Tran, 2020). The pulmonary problems of CF result from the constant presence of thick, sticky mucus and are the most serious complications of the disease. The mucus narrows airways, reducing airflow and interfering with gas exchange . The constant presence of mucus results in chronic respiratory tract infections, chronic bronchitis, and dilation of the bronchioles (bronchiectasis). Lung abscesses are common. Over time the bronchioles distend, and mucus-producing cells have increased numbers (hyperplasia) and increased size (hypertrophy). Complications include pneumothorax, arterial erosion and hemorrhage, and respiratory failure. CF is most common among whites, and about 4% (1 in 29) are carriers (CFF, 2019). It is rarer among Hispanic Americans (1 in 46 are carriers), African Americans (1 in 65 are carriers), and Asian American (1 in 90 are carriers). Males and females are affected equally Interprofessional Collaborative Care Assessment: Recognize Cues Usually, but not always, CF is diagnosed in childhood. The major diagnostic test is sweat chloride analysis. Non-pulmonary symptoms include abdominal distention, gastroesophageal reflux, rectal prolapse, foul-smelling stools, and steatorrhea (excessive fat in stools). The patient is often malnourished and has many vitamin deficiencies, especially of the fat-soluble vitamins (e.g., vitamins A, D, E, K). As pancreatic function decreases, diabetes mellitus develops with loss of insulin production. Diabetes is present in more than 50% of adults with CF (Frost et al., 2018). The adult with severe CF is usually smaller and thinner than average. Another problem seen in adults with CF is the early onset of osteoporosis and osteopenia, with a greatly increased risk for bone fracture (CFF, 2019). Pulmonary symptoms caused by CF are progressive (McCance et al., 2019). Respiratory infections are frequent or chronic with exacerbations. Patients usually have chest congestion, limited exercise tolerance, cough, sputum production, use of accessory muscles, and decreased pulmonary function (especially forced vital capacity [FVC] and forced expiratory volume in the first second of exhalation [ FEV1 ]). Chest x-rays show infiltrate and an increased anteroposterior (AP) diameter. During an acute exacerbation or when the disease progresses to end stage, the patient has increased chest congestion, reduced activity tolerance, increased crackles, increased cough, increased sputum production (often with hemoptysis), and severe dyspnea with fatigue. Arterial blood gas (ABG) studies show acidosis (low pH), greatly reduced arterial oxygen (PaO 2 ) levels, increased arterial carbon dioxide (PaCO 2 ) levels, and increased bicarbonate levels. Interventions: Take Action The patient with CF needs daily therapy to slow disease progress and enhance gas exchange . There is no cure for CF. Nonsurgical Management The management of the patient with CF is complex and lifelong. Nutrition management focuses on weight maintenance, vitamin supplementation, diabetes management, and pancreatic enzyme replacement (Razga & Handu, 2019). Pulmonary management focuses on preventive maintenance and management of exacerbations. Priority nursing interventions focus on teaching about drug therapy, infection prevention, pulmonary hygiene, nutrition, and vitamin supplementation. Preventive/maintenance therapy involves the use of positive expiratory pressure, active cycle of breathing technique, and an individualized exercise program. Daily chest physiotherapy with postural drainage is beneficial for the patient with CF. This therapy uses chest percussion, chest vibration, and dependent drainage to loosen secretions and promote drainage. Increasingly, the use of a chest physiotherapy (CPT) vest is recommended (Fig. 27.9), although no specific evidence supports its use as superior to any other type of chest physiotherapy (Wilson, 2018). This system uses an inflatable vest that rapidly fills and deflates, gently compressing and releasing the chest wall up to 25 times per second, a process called high-frequency chest wall oscillation (HFCWO). The action creates minicoughs that dislodge mucus from the bronchial walls, increase mobilization, and move it toward central airways, where it can be removed by coughing or suctioning. HFCWO also thins secretions, making them easier to clear. Pulmonary function tests are monitored regularly. Daily drugs include bronchodilators, antiinflammatories, mucolytics, and antibiotics. Exacerbation therapy is needed when the patient with CF has increased chest congestion, reduced activity tolerance, increased or new-onset crackles, and a 10% decrease in FEV1 . Other symptoms include increased sputum production with bloody or purulent sputum, increased coughing, decreased appetite, weight loss, fatigue, decreased SpO 2 , and chest muscle retractions. Often infection is present, with fever, increased lung infiltrate on x-ray, and an elevated white blood cell count Gene therapy for CF is available for use in patients with specific gene mutations. The drug ivacaftor, known as a CFTR modulator or potentiator, has been found to be of value to patients with CF who are heterozygous for any one of about 35 specific mutations in the CFTR gene alleles. It is of no benefit to patients who are homozygous for the mutations (Coulthard, 2018). This drug helps improve chloride transport by increasing the time that the ion channels are open. The combination drug ivacaftor/lumacaftor is effective as therapy for patients whose CF is caused by the F508del (also known as the Phe508del) mutation, the most common mutation involved in CF, even in patients who are homozygous for the mutation with both alleles being affected. This oral drug combination is considered a “corrector” rather than a modulator, by moving the activated CFTR to the membrane surface for improved function. Another newly approved corrector oral drug, tezacaftor, when combined with ivacaftor has some effect for patients with the Phe508del mutation and appears to have fewer adverse respiratory events than the other combination, although decreases in liver function have been found with the use of all of these drugs. The successful outcome for these agents is increased movement of chloride ions across epithelial membranes, resulting in reduced sodium and fluid absorption so that mucus is less thick and sticky. These drugs have no effect in patients whose CFTR gene does not have the specific mutations. A significant drawback to these therapies is the cost, which is about $250,000/year of treatment (Coulthard, 2018). Surgical Management Surgical management of the patient with CF is lung transplantation. The patient has greatly reduced symptoms but is at continuing risk for lethal pulmonary infections, especially with antirejection drug therapy. Nonpulmonary problems are not helped by this treatment. Transplantation extends life for 1 to 15 years with an average of 7 years, but the transplant rejection rate is high, possibly caused by poor GI absorption of antirejection drugs (CFF, 2019). Fewer lung transplants are performed compared with transplantation of other solid organs because of the scarcity of available lungs. In addition, many patients who could benefit from lung transplantation have serious problems in other organs that make the procedure even more dangerous. Lung transplant procedures include two lobes or a single lung transplantation, as well as double-lung transplantation. The type of procedure is determined by the patient’s overall condition and the life expectancy after transplantation. Usually the patient with CF has a bilateral lobe transplant from either a cadaver donor or a living-related donor. Pulmonary Arterial Hypertension (PAH): • • • • Pulmonary vessels and often other lung tissues undergo growth changes that greatly increase pressure in the lung circulatory system for unknown reasons Dyspnea, fatigue Drug therapy, oxygen Surgical management • Lung transplantation • Heart-lung transplantation (if cor pulmonale) Pulmonary Arterial Hypertension Pathophysiology Review Pulmonary hypertension is the chronic increase in pulmonary vascular pressures above 25 mm Hg, which makes the right side of the heart work much harder for lung perfusion to support proper gas exchange . Normally the pulmonary vascular pressures are low, 15 to 18 mm Hg, allowing the pressures generated by the contraction of the less-muscled right ventricle to easily overcome them and move blood into the pulmonary artery (McCance et al., 2019). Over time, the higher lung vascular pressures lead to right-sided heart failure (cor pulmonale) that can be fatal. Many lung problems, such as chronic obstructive pulmonary disease and pulmonary fibrosis, can secondarily cause increased pulmonary pressures. For secondary pulmonary hypertension, good control of the condition causing it can delay or prevent cor pulmonale. Primary pulmonary artery hypertension (PAH), also known as idiopathic pulmonary artery hypertension, is a condition in which pulmonary vessels and often other lung tissues undergo growth changes that greatly increase pressure in the lung circulatory system for unknown reasons. Just as with secondary pulmonary hypertension, PAH progresses and leads to cor pulmonale with reduced perfusion and gas exchange . The absolute cause of PAH is unknown, and it is diagnosed in the absence of other lung disorders (McCance et al., 2019). Genetic and environmental factors working together may increase the risk. For example, although it is a relatively rare problem, exposure to some drugs, such as fenfluramine/phentermine or dasatinib, increases the risk. The disorder occurs mostly in women between the ages of 20 and 40 years and occurs more often within some families, suggesting a possible genetic susceptibility. The familial PAH form appears to be transmied in an autosomal dominant paern with reduced penetrance (OMIM, 2018). Without treatment, death usually occurs within 2 years after diagnosis, most often from profound heart failure. The pathologic problem in PAH is blood vessel constriction with increasing vascular resistance in the lung. The events that lead up to increasing resistance may include an imbalance between those factors that increase vascular resistance and those that induce blood vessel relaxation. Intrinsic agents that increases vascular resistance are endothelin-1, a very powerful vasoconstrictor that works by binding to endothelin receptors on vascular smooth muscle, and thromboxane, which induces arterial vasoconstriction and enhances cloing by activating platelets. Intrinsic factors that promote vascular relaxation are nitric oxide (NO) and prostacyclin-1. Many adults with primary PAH have a deficiency of prostacyclin 1 • Pathophysiology overview • Poor long-term survival • SCLC and NSCLC • Metastasis • Paraneoplastic syndromes • Staging Lung Cancer Pathophysiology Review Lung cancer is a leading cause of cancer-related deaths worldwide in affluent and less affluent countries. In North America, more deaths from lung cancer occur each year than from prostate cancer, breast cancer, and colon cancer combined, although the incidence has been decreasing somewhat for the past decade. In the United States, more than 228,000 new cases are diagnosed each year and more than 135,000 deaths occur from lung cancer annually (American Cancer Society [ACS], 2020). In Canada, more than 29,300 new cases are diagnosed each year and more than 21,000 deaths from lung cancer occur annually (Canadian Cancer Society, 2019). The overall 5-year survival for all patients with lung cancer is only about 19%. This poor long-term survival is because most lung cancers are diagnosed at a late stage, when metastasis is present. Only 15% of patients have small tumors and localized disease at the time of diagnosis. The 5- year survival rate for this population is 56% (ACS, 2020). The prognosis for advanced lung cancer remains poor. Treatment often focuses on relieving symptoms or increasing survival time (palliation) rather than cure. Most primary lung cancers arise as a result of failure of cellular regulation in the bronchial epithelium. Chapter 19 discusses the general mechanisms and processes of cancer development. Lung cancers are collectively called bronchogenic carcinomas and are classified as small cell lung cancer (SCLC) and non– small cell lung cancer (NSCLC). NSCLC has several subtypes that are managed in the same ways, although causes and locations in the lungs differ. Metastasis (spread) of lung cancer occurs by direct extension, through the blood, and by invading lymph glands and vessels. Tumors in the lungs can grow and obstruct the bronchus partially or completely, interfering with gas exchange . Tumors in other areas of lung tissue can grow so large that they can compress and obstruct the airway. Compression of the alveoli, nerves, blood vessels, and lymph vessels can occur and also interfere with gas exchange . Lung cancer can spread to the lung lymph nodes, distant lymph nodes, and other tissues including bone, liver, brain, and adrenal glands. Additional symptoms, known as paraneoplastic syndromes, complicate certain lung cancers. The paraneoplastic syndromes are caused by hormones secreted by tumor cells and occur most commonly with SCLC. Table 27.4 lists the endocrine paraneoplastic syndromes that may occur. Staging of lung cancer is performed to assess the size and extent of the disease using the standard TNM system described in Chapter 19. Higher numbers represent later stages and less chance for cure or longterm survival. Incidence and Prevalence Lung cancers occur as a result of repeated exposure to inhaled substances that cause chronic tissue irritation or inflammation interfering with cellular regulation of cell growth. Cigarette smoking is the major risk factor and is responsible for 81% of all lung cancer deaths (ACS, 2020). Etiology and Genetic Risk: Nonsmokers exposed to “secondhand” or “thirdhand” smoke also have a greater risk for lung cancer than do nonsmokers who are minimally exposed to cigarette smoke. See Chapter 24 for a discussion of passive smoking risks. Other lung cancer risk factors include chronic exposure to asbestos, beryllium, chromium, coal distillates, cobalt, iron oxide, mustard gas, petroleum distillates, radiation, tar, nickel, and uranium. Air pollution with benzopyrenes and hydrocarbons also increases the risk for lung cancer. Lung cancer, especially the adenocarcinoma form of NSCLC, does occur in adults who are “never smokers” particularly among women. Possible contributing factors for lung cancer in this population include exposure to environmental carcinogens, second-hand smoke exposure, genetic differences, familial predisposition, and advancing age. Interprofessional Collaborative Care Assessment: Recognize Cues History: Ask the patient about risk factors, including smoking, hazards in the workplace, and warning signals (Table 27.5). Calculate the pack-year smoking history as described in Chapter 24. Ask about the presence of lung cancer symptoms, such as hoarseness, cough, sputum production, hemoptysis, shortness of breath, or change in endurance. Symptoms often have been present for years. Ask the patient to describe any recent symptom changes or if position affects them. Assess for chest pain or discomfort, which can occur at any stage of tumor development. Chest pain may be localized or on just one side and can range from mild to severe. Ask about any sensation of fullness, tightness, or pressure in the chest, which may suggest obstruction. A piercing chest pain or pleuritic pain may occur on inspiration. Pain radiating to the arm results from tumor invasion of nerve plexuses in advanced disease. Physical Assessment/Signs and Symptoms—Pulmonary Symptoms of lung cancer are often nonspecific and appear late in the disease. Specific symptoms depend on tumor location. Chills, fever, and cough may be related to pneumonitis or bronchitis that occurs with obstruction. Assess sputum quantity and character. Blood-tinged sputum may occur with bleeding from a tumor. Hemoptysis is a later finding in the course of the disease. If infection or necrosis is present, sputum may be purulent and copious. Breathing may be labored or painful. Obstructive breathing may occur as prolonged exhalation alternating with periods of shallow breathing. Rapid, shallow breathing occurs with pleuritic chest pain and an elevated diaphragm. Look for and document abnormal retractions, the use of accessory muscles, flared nares, stridor, and asymmetric diaphragmatic movement on inspiration. Dyspnea and wheezing may be present with airway obstruction. Ask about dyspnea severity at rest, with activity, and in the supine position. Assess how much the dyspnea interferes with the patient’s participation in ADLs, work, recreational activities, and family responsibilities. Increased vibrations felt on the chest wall when the patient speaks (fremitus) indicate areas of the lung where air spaces are replaced with tumor or fluid. Fremitus is decreased or absent when the bronchus is obstructed. The trachea may be displaced from midline if a mass is present in the area. Breath sounds may change with the presence of a tumor. Wheezes indicate partial obstruction of airflow in passages narrowed by tumors. Decreased or absent breath sounds indicate complete obstruction of an airway by a tumor or fluid. A pleural friction rub may be heard when inflammation is present Physical Assessment/Signs and Symptoms—Non-pulmonary Many other systems can be affected by lung cancer and have changes at the time of diagnosis. Heart sounds may be muffled by a tumor or fluid around the heart (cardiac tamponade). Dysrhythmias may occur as a result of hypoxemia or direct pressure of the tumor on the heart. Cyanosis of the lips and fingertips or clubbing of the fingers may be present (see Fig. 27.8). Bones lose density with tumor invasion and break easily with lile pressure and without trauma. The patient may have bone pain or fragility fractures. Late symptoms of lung cancer usually include fatigue, weight loss, anorexia, dysphagia, and nausea and vomiting. Superior vena cava syndrome may result from tumor pressure in or around the vena cava. This syndrome is an emergency (see Chapter 20 ) and requires immediate intervention. The patient may have confusion or personality changes from brain metastasis. Psychosocial Assessment The poor prognosis for lung cancer has made it a much-feared disease. Dyspnea and pain add to the patient’s fear and anxiety. Encourage the patient and family to express their feelings about the possible diagnosis of lung cancer. Diagnostic Assessment Most commonly, lung lesions are first identified on chest x-rays. CT scans are then used to identify the lesions more clearly and guide biopsy procedures. The definitive diagnosis of lung cancer is made by examination of cancer cells from biopsy or from pleural effusion fluid (if present). A thoracoscopy to directly view lung tissue may be performed through a video-assisted thoracoscope entering the chest cavity via small incisions through the chest wall. Other diagnostic studies may be needed to determine how widely the cancer has spread. Such tests include MRI and radionuclide scans of the liver, spleen, brain, and bone help. Positron emission tomography (PET) scanning is a thorough way to locate metastases. These tests help determine the extent of the cancer and the best methods to treat it. Non-surgical Chemotherapy is often the treatment of choice for lung cancers, especially small cell lung cancer (SCLC). It may be used alone or as adjuvant (addon) therapy in combination with surgery for non–small cell lung cancer (NSCLC). The combination of drugs used depends on tumor response and the overall health of the patient; however, most include platinum-based agents. Side effects of cancer chemotherapy include chemotherapy-induced nausea and vomiting (CINV), alopecia (hair loss), open sores on mucous membranes (mucositis), immunosuppression with neutropenia, anemia, decreased numbers of platelets, and peripheral neuropathy. Consult Chapter 20 for discussion of the nursing care needs for patients who have these side effects. Immunosuppression with neutropenia, which greatly increases the risk for infection, is the major dose-limiting side effect of chemotherapy for lung cancer. It can be managed by the use of growth factors to stimulate bone marrow production of immune system cells. Teach the patient and family about precautions to take to reduce the patient’s risk for infection (see Chapter 20 for information about chemotherapy and associated nursing care). Targeted therapy is common in the treatment of non−small cell lung cancer (NSCLC). These agents take advantage of one or more differences in cancer cell growth or metabolism that is either not present or only slightly present in normal cells. For lung cancer, usually these differences are identified as variations in two different genes, EGFR (which codes for epidermal growth factor receptors) and ALK (which codes for ALK receptor tyrosine kinases). Agents used as targeted therapies work to disrupt cancer cell division in one of several ways. However, these agents only work when the cancer cell has the particular target or specific genetic mutation. Therefore testing of the cancer cells is needed before therapy begins, and not all cancers of the same type express the target. These agents are increasing survival time for patients with NSCLC but do not lead to a cure. The Common Examples of Drug Therapy: Targeted Therapy and Immunotherapy of Lung Cancer box lists some targeted agents used in lung cancer therapy. Immunotherapy for lung cancer is a type of targeted therapy designed to allow the patient’s own immune system to beer recognize and aack his or her cancer cells. Normally, certain immune system cells including Tcells, B-cells, monocytes, and natural killer (NK) cells recognize and aack foreign cells and unhealthy self cells. Radiation therapy can be an effective treatment for locally advanced lung cancers confined to the chest. Best results are seen when radiation is used in addition to surgery or chemotherapy. Radiation may be performed before surgery to shrink the tumor and make resection easier. Photodynamic therapy (PDT) may be used to remove small bronchial tumors when they are accessible by bronchoscopy. This therapy first involves injecting the patient with an agent that sensitizes cells to light and remains in cancer cells longer than normal cells. After 48 to 72 hours, most of the drug has accumulated in the cancer cells. A laser light is focused on the tumor with the patient intubated and under anesthesia. The light activates a reaction that causes irreversible damage and death to cells retaining the sensitizing drug. surgical Management Surgery is the main treatment for stage I and stage II NSCLC. Total tumor removal may result in a cure. If complete resection is not possible, the surgeon removes the bulk of the tumor. The specific surgery depends on the stage of the cancer and the patient’s overall health. Lung cancer surgery may involve removal of the tumor only, removal of a lung segment, removal of a lobe (lobectomy), or removal of the entire lung (pneumonectomy). These procedures can be performed by open thoracotomy or by minimally invasive surgery in select patients. Preoperative care is focused on relieving anxiety and promoting the patient’s participation (see Chapter 9 for routine preoperative care). Reinforce the surgeon’s explanation of the procedure, and provide education related to what is expected after surgery. Teach about the probable placement of the chest tube and drainage system (except after pneumonectomy). Operative procedures for lung cancer may consist of a lobectomy, pneumonectomy, segmental resection, or wedge resection. A segmental resection is a lung resection that includes the bronchus, pulmonary artery and vein, and tissue of the involved lung segment or segments of a lobe. A wedge resection is removal of the peripheral portion of small, localized areas of disease. A lobe or entire lung can be removed through video-assisted thoracoscopic surgery (VATS), which is minimally invasive, in select patients. The procedure involves making small incisions in the chest for placement of the instruments. The lung, section, or lobe is then isolated from its airway, which is surgically closed. The lobe or lung is closed off from the rest of the lung and sealed in a bag to prevent leakage of tumor tissue and possible seeding of the cancer. The bagged lung is then removed whole through one of the small incisions. Postoperative care for patients who have undergone thoracotomy (except for pneumonectomy) requires closed-chest drainage to drain air and blood that collect in the pleural space. A chest tube drain placed in the pleural space allows lung re-expansion and prevents air and fluid from returning to the chest (Fig. 27.10). The drainage system consists of one or more chest tubes or drains, a collection container placed below the chest level, and a water seal to keep air from entering the chest. The drainage system may be a stationary, disposable, self-contained system (Fig. 27.11) or a smaller, portable, disposable, self-contained system that requires no connection to a vacuum source (Fig. 27.12). The nursing care priorities for the patient with a chest tube are to ensure the integrity of the system, promote comfort, ensure chest tube patency, and prevent complications (Sasa, 2019). Chest Tube Placement and Care The tip of the tube used to drain air is placed near the front lung apex (see Fig. 27.10). The tube that drains liquid is placed on the side near the base of the lung. The wounds are covered with airtight dressings, most commonly silicone foam dressings (Wood et al., 2019). The chest tube is connected by about 6 feet of tubing to a collection device placed below the chest, allowing gravity to drain the pleural space while the patient can turn and move without pulling on the chest tube. When two chest tubes are inserted, they are joined by a Yconnector close to the patient and the 6 feet of tubing is aached to the Y-connector. Stationary chest tube drainage systems, such as the Pleur-evac system, use a water-seal mechanism that acts as a one-way valve to prevent air or liquid from moving back into the chest cavity. Disposable system use a one-piece disposable plastic unit with three chambers. The three chambers are connected to one another. The tube(s) from the patient is (are) connected to the first chamber in the series of three, which is the drainage collection container. The second chamber is the water seal to prevent air from moving back up the tubing system and into the chest. The third chamber, when suction is applied, is the suction regulator. Tubing from the patient penetrates chamber one shallowly, as does the tube connecting chamber one with chamber two. The fluid in chamber one collected from the patient is measured hourly during the first 24 hours. This drainage fluid must never fill to the point that it comes into contact with any tubes! If the tubing from the patient enters the fluid, drainage stops and can lead to a tension pneumothorax. Chamber two is the water seal that prevents air from re-entering the patient’s pleural space. As the trapped air leaves the pleural space, it will pass through chamber one (collection chamber) before entering chamber two (the water-seal chamber), which should always contain at least 2 cm of water to prevent air from returning to the patient. As trapped air from the patient’s pleural space passes through the water seal, which serves as a one-way valve, the water will bubble. Once all the air has been evacuated from the pleural space, bubbling of the water seal stops. Pain Management Pain control measures are needed regardless of whether surgery is performed as an open procedure or with minimally invasive techniques. Give the prescribed drugs for pain and assess the patient’s responses to them. Teach patients using patient-controlled analgesia (PCA) devices to self-administer the drug before pain intensity becomes too severe. Monitor vital signs before and after giving opioid analgesics, especially for the patient who is not being mechanically ventilated. Respiratory Management Immediately after surgery the patient is mechanically ventilated. See Chapter 29 for nursing care of the patient receiving mechanical ventilation. Once the patient is breathing on his or her own, the priorities are to maintain a patent airway, ensure adequate ventilation, and prevent complications. Assess the patient at least every 2 hours for adequacy of ventilation and gas exchange . Check the alignment of the trachea. Assess oxygen saturation and the rate and depth of respiration. Listen to breath sounds on the nonoperative side, particularly noting the presence of crackles. Perform oral suctioning only as needed. Usually the patient receives oxygen by mask or nasal cannula for the first 2 days after surgery. Assist the patient to a semi-Fowler position or up in a chair as soon as possible. Encourage him or her to use the incentive spirometer every hour while awake. If coughing is permitted, help him or her cough by splinting any incision and ensuring that the chest tube does not pull with movement. Specially designed walkers that support the patient and all equipment for early ambulation with chest tubes in place are available (Grondell et al., 2018). Interventions for Palliation Oxygen therapy is prescribed when the patient is hypoxemic and helps relieve dyspnea and anxiety. (See Chapter 25 for issues related to home oxygen therapy.) Radiation therapy can help relieve hemoptysis, obstruction of the bronchi and great veins (superior vena cava syndrome), difficulty swallowing from esophageal compression, and pain from bone metastasis. Radiation for palliation uses higher doses for shorter periods. Thoracentesis is performed when pleural effusion is a problem for the patient with lung cancer. The excess fluid increases dyspnea, discomfort, and the risk for infection. The purpose of treatment is to remove pleural fluid and prevent its formation. Thoracentesis is a procedure for fluid removal by suction after the placement of a large needle or catheter into the intrapleural space. Fluid removal temporarily relieves hypoxia; however, the fluid can rapidly reform in the pleural space. When fluid development is continuous and uncomfortable, a tunneled pleural catheter that continuously drains may be placed into the intrapleural space to collect the fluid (Miller et al., 2018). Dyspnea management is needed because the patient with lung cancer tires easily and is often most comfortable resting in a semi-Fowler position. Dyspnea is reduced with oxygen, use of a continuous morphine infusion, and positioning for comfort. The severely dyspneic patient may be most comfortable siTTing and sleeping in a lounge chair or reclining chair. Pain management is usually to help the patient be as pain free and comfortable as possible. Pharmacologic management with opioid drugs as oral, parenteral, or transdermal preparations is needed. Analgesics are most effective when given around the clock with additional PRN analgesics used for breakthrough pain. Hospice care can be beneficial for the patient in the terminal phase of lung cancer. Hospice programs provide support to the terminally ill patient and the family, meet physical and psychosocial needs, adjust the palliative care regimen as needed, make home visits, and provide volunteers for errands and respite care. (See Chapter 8 for a more complete discussion of end-of life issues.) Concepts of Care for Patients With Infectious Respiratory Problems anergy Failure to have a skin response to TB skin testing because of reduced immunity even when infection is present. consolidation: An abnormal solidification with lack of air spaces in a segment of area of the lung. COVID-19: A new coronavirus mutation (CO= corona; VI = virus; D = disease; 19 = 2019, the year the new virus was identified) that enabled this animal virus to infect humans and is responsible for the 2020 influenza pandemic. empyema: A collection of pus in the pleural cavity. endemic infection: Respiratory infection caused by organisms that are much more common within a geographic location but the actual incidence of the infection is relatively low. gas exchange: Oxygen transport to the cells and carbon dioxide transport away from cells through ventilation and diffusion. immunity Protection from illness or disease that is maintained by the body’s physiologic defense mechanisms. induration Localized swelling with hardness of soft tissue. infection Invasion of pathogens into the body that multiply and cause disease or illness. inflammation A syndrome of normal tissue responses to cellular injury, allergy, or the invasion of pathogens. miliary (hematogenous) TB :Spread of TB throughout the body when a large number of organisms enter the blood. pandemic infection: An infection with an organism to which most humans have no immunity and that has the potential to spread globally. tuberculosis (TB) A highly communicable disease caused by infection with Mycobacterium tuberculosis Seasonal Influenza Pathophysiology Review Seasonal influenza, or “flu,” is a highly contagious acute viral respiratory infection that can occur at any age (Cannon et al., 2018). Influenza may be caused by different strains of one of several virus families, referred to as A, B, and C. Epidemics are common and lead to complications of pneumonia or death, especially in older adults, those with heart failure or chronic lung disorders, and immunocompromised patients. Most patients are treated at home, but hospitalization may be needed when symptoms are severe or the patient develops complications such as pneumonia. During the 2017−2018 influenza season, more than 30,000 patients were hospitalized for the infection with about 60% of these being older than 65 years (Centers for Disease Control and Prevention [CDC], 2018d). The patient with influenza often has a rapid onset of severe headache, muscle aches, fever, chills, fatigue, and weakness. Adults are contagious 24 hours before symptoms occur and up to 5 days after they begin. Sore throat, cough, and watery nasal discharge can also occur. Infection with influenza strain B can lead to nausea, vomiting, and diarrhea. Most patients feel fatigued for 1 to 2 weeks after the acute episode has resolved Highly contagious acute viral respiratory infection Rapid onset of severe headache, muscle ache, fever, chills, fatigue, weakness, anorexia Preventable (or severity is reduced) with vaccination Handwashing is critical Antiviral agents may be effective if started within 24 to 48 hours of symptoms Pandemic Influenza A pandemic respiratory viral infection is one that has the potential to spread globally because the virus has previously infected only birds or other animals, so no human ancestral immunity is present. Most bird and animal viruses cannot be transmitted to humans. A few notable exceptions have occurred when these viruses mutated and became highly infectious to humans, causing pandemics. Example pandemics include the 1918 “Spanish” influenza and the 2009 H1N1 influenza A. Potential to spread globally Avian flu, MERS, SARS Early recognition and quarantine Contact and Airborne Precautions (until specific type of pandemic influenza is identified with routes of transmission known) Antiviral drugs such as oseltamivir are stockpiled in the event of a pandemic influenza. They can be used for prevention or to shorten the duration of the infection. Distribution for treatment is made on a case-byc2ase basis, as the drug must be started within 48 hours of symptom onset. Infected patients in the hospital seing should be placed on Droplet Precautions for 7 days and placed in a private room. Etiology: Infectious pneumonia develops when a patient’s immunity cannot overcome the invading organisms (Arsbad et al., 2016). Organisms from the environment (especially after natural disasters), invasive devices, equipment, and supplies or other people can invade the body. Risk factors are listed in Table 28.1. Pneumonia can be caused by any organism such as bacteria, viruses, mycoplasmas, fungi, rickesiae, protozoa, and helminths (worms). Noninfectious causes of pneumonia include inhalation of toxic gases, chemical fumes, and smoke; and aspiration of water, food, fluid (including saliva), and vomitus. Infectious pneumonia can be categorized as community acquired (CAP), hospital acquired (HAP), health care acquired (HCAP) or ventilator-associated (VAP) (see Table 28.2). Incidence and Prevalence In the United States 2 to 5 million cases of pneumonia occur annually. About 1 million people are hospitalized for treatment, and more than 50,000 deaths result from the disease (CDC, 2018b). In Canada, influenza and pneumonia incidence and deaths are reported together and both disorders are common, accounting for about 6000 deaths annually (Statistics Canada, 2018). The rate of pneumonia is higher among older adults, nursing home residents, hospitalized patients, patients with neurologic problems or difficulty swallowing, and those being mechanically ventilated (Meehan & McKenna, 2020). Health Promotion and Maintenance Vaccination can help prevent pneumonia. Currently, there are two pneumonia vaccines: pneumococcal polysaccharide vaccine (PPSV23) and pneumococcal conjugate vaccine (PCV13) for prevention of pneumonia (Phillips & Swanson, 2016). The CDC recommends that adults older than 65 years be vaccinated with both, first with PCV13 followed by PPSV23 about 12 months later. Adults who have already received the PPSV23 should have PCV13 about a year or more later. These recommendations also apply to adults between 19 and 64 years of age who have specific risk factors such as chronic illnesses (CDC, 2018b). Because pneumonia often follows influenza, especially among older adults, urge all adults to receive the seasonal vaccination annually. Patient education about vaccination and other means of pneumonia prevention is important. Teaching points are presented in the Patient and Family Education: Preparing for Self-Management: Preventing Pneumonia box Other pneumonia prevention techniques include strict handwashing to avoid spreading organisms and avoiding crowds during cold and flu season. Teach the patient who has a cold or the flu to see his or her primary health care provider if fever lasts more than 24 hours, the problem lasts longer than 1 week, or symptoms worsen. Respiratory therapy equipment must be well maintained and decontaminated or changed as recommended. Use sterile water rather than tap water in GI tubes and institute Aspiration Precautions as indicated, including screening patients for aspiration risk (Meehan & McKenna, 2020). VAP is on the rise, but the risk can be reduced with conscientious assessment and meticulous nursing care. The preventive care for VAP is discussed in Chapter 29 and is listed in Table 28.2 Vaccination Avoid crowded places during flu season Cough, turn, move, deep breathe Clean respiratory equipment Avoid pollutants Stop smoking Get rest and sleep Eat healthy diet Drink 3L of water daily (unless fluids are restricted) History Assess for the risk factors for respiratory infection (see Table 28.1). Document age; living, work, or school environment; diet, exercise, and sleep routines; swallowing problems; presence of a nasogastric tube; tobacco and alcohol use; and past and current use of or addiction to “street” drugs. Remember that often aspiration is “silent” with no signs or symptoms. Ask about past respiratory illnesses and whether the patient has been exposed to influenza or pneumonia or has had a recent viral infection. Using the I PREPARE model listed in Chapter 24, assess the patient for particulate maer exposure (PME). Even noninfectious exposures can result in respiratory inflammation , which increases the risk for pneumonia development. If the patient has chronic respiratory problems, ask whether respiratory equipment is used in the home. Assess whether the patient’s cleaning routine for the equipment is adequate to prevent infection. Ask when he or she received the last influenza or pneumococcal vaccine. Ask family members whether they have noticed a change in the patient’s cognition . Physical Assessment/Signs and Symptoms: Observe the general appearance. Many patients with pneumonia have flushed cheeks and an anxious expression. The patient may have chest pain or discomfort, myalgia, headache, chills, fever, cough, tachycardia, dyspnea, tachypnea, hemoptysis (bloody sputum), and sputum production. Severe chest muscle weakness may also be present from sustained coughing. Observe the patient’s breathing pattern, position, and use of accessory muscles. The patient with hypoxia and reduced gas exchange may be uncomfortable in a lying position and will sit upright, balancing with the hands (“tripod position”). Assess the cough and the amount, color, consistency, and odor of sputum produced. Crackles are heard on auscultation when fluid is in interstitial and alveolar areas, and breath sounds may be diminished. Wheezing may be heard if inflammation or exudate narrows the airways. Bronchial breath sounds are heard over areas of density or consolidation. Fremitus is increased over areas of pneumonia, and percussion is dulled. Chest expansion may be diminished or unequal on inspiration. In evaluating vital signs, compare the results with baseline values. A patient with pneumonia, especially an older adult, is often hypotensive with orthostatic changes because of vasodilation and dehydration. A rapid, weak pulse may indicate hypoxemia, dehydration, or impending sepsis and shock. Dysrhythmias may occur from cardiac tissue hypoxia. Common pneumonia signs and symptoms and their causes are listed in Table 28.3. The primary health care provider uses one of several evidence-based pneumonia severity scales to determine whether the patient can be managed in the community or requires hospitalization. When pneumonia is uncomplicated by other health problems, it is often managed in the community. Psychosocial Assessment The patient with pneumonia often has pain, fatigue, and dyspnea, all of which promote anxiety. Assess anxiety by looking at his or her facial expression and general tenseness of facial and shoulder muscles. Listen to the patient carefully, and use a calm approach. Because of airway obstruction and muscle fatigue, the patient with dyspnea speaks in broken sentences. Keep the interview short if severe dyspnea or breathing discomfort is present. Laboratory Assessment Sputum is obtained and examined by Gram stain, culture, and sensitivity testing; however, the responsible organism is often not identified. A sputum sample is easily obtained from the patient who can cough into a specimen container. Extremely ill patients may need suctioning to obtain a sputum specimen. In these situations, a specimen is obtained by sputum trap (Fig. 28.1) during suctioning. A complete blood count (CBC) is obtained to assess for an elevated WBC count, which is a common finding except in older adults. Blood cultures may be performed to determine whether the organism has entered the bloodstream. In severely ill patients, arterial blood gases (ABGs) may be assessed to determine baseline arterial oxygen and carbon dioxide levels and to help identify a need for supplemental oxygen. Serum electrolyte, blood urea nitrogen (BUN), and creatinine levels are also assessed. A high BUN level may occur as a result of dehydration. Hypernatremia (high blood sodium levels) occurs with dehydration. A lactate level may be performed to help assess for sepsis. Because CAP often follows or is present with influenza, recommend that adults with CAP also have influenza testing (Esden, 2020). Imaging Assessment Chest x-ray is the most common diagnostic test for pneumonia but may not show changes until 2 or more days after symptoms are present. Pneumonia usually appears on chest x-ray as an area of increased density. It may involve a lung segment, a lobe, one lung, or both lungs. In the older adult, the chest x-ray is essential for early diagnosis because other pneumonia symptoms are often vague Other Diagnostic Assessments Pulse oximetry is used to assess for hypoxemia. Thoracentesis is used in patients who have an accompanying pleural effusion. Analysis: Analyze Cues and Prioritize Hypotheses The priority interprofessional collaborative problems for patients with pneumonia include: 1. Decreased gas exchange due to decreased diffusion at the alveolarcapillary membrane 2. Potential for airway obstruction due to inflammation with excessive pulmonary secretions, fatigue, muscle weakness 3. Potential for sepsis due to the presence of microorganisms in a very vascular area and reduced immunity 4. Potential for pulmonary empyema due to spread of infectious organisms from the lung into the pleural space Care Coordination and Transition Management The patient needs to continue the anti-infective drugs as prescribed. An important nursing role is to reinforce, clarify, and provide information to the patient and family as needed. Unfortunately, the readmission rate within 30 days after discharge is relatively high, especially among older adults (Goering, 2018). See the accompanying Systems Thinking and Quality Improvement box focusing on one group’s successful method of reducing preventable re-admissions. Home Care Management No special changes are needed in the home. If the home has a second story, the patient may prefer to stay on one floor for a few weeks, because stair climbing can be tiring. Toileting needs may be met by using a bedside commode if a bathroom is not located on the level the patient is using. Home care needs depend on the patient’s level of fatigue, dyspnea, and family and social support. The long recovery phase, especially in the older adult, can be frustrating. Fatigue, weakness, and a residual cough can last for weeks. Some patients fear they will never return to a “normal” level of functioning. Prepare them for the disease course and offer reassurance that complete recovery will occur. After discharge a home nursing assessment may be helpful. Specific issues to assess for a patient recovering from pneumonia are presented in the Focused Assessment box. Self-Management Education Review all drugs with the patient and family and emphasize the importance of completing anti-infective therapy. Instruct the patient to notify the primary health care provider if chills, fever, persistent cough, dyspnea, wheezing, hemoptysis, increased sputum production, chest discomfort, or increasing fatigue returns or fails to go away completely. Instruct him or her to get plenty of rest and increase activity gradually. An important aspect of education for the patient and family is avoiding upper respiratory tract infection and viruses. Teach him or her to avoid crowds (especially in the fall and winter when viruses are prevalent), people who have a cold or flu, and exposure to irritants such as smoke. A balanced diet and adequate fluid intake are essential. Health Care Resources Inform patients who smoke or vape that these activities are risk factors for pneumonia. Use the suggestions and interventions discussed in Chapter 24 to help the patient quit or reduce cigarette smoking and/or vaping. Teach about pneumonia, and urge the patient who has not already been vaccinated against influenza or pneumonia to get these vaccinations after the pneumonia has resolved. Evaluation: Evaluate Outcomes Evaluate the care of the patient with pneumonia based on the identified priority patient problems. The expected outcomes are that he or she: • Attains or maintains adequate gas exchange with SaO 2 of at least 95% or his or her normal level • Maintains patent airways as evidenced by absence of crackles and wheezes on auscultation • Is free from infection as evidenced by absence of fever and a WBC count within normal limits • Avoids empyema • Returns to his or her pre-pneumonia health status Infection Concept Exemplar: Pulmonary Tuberculosis Pathophysiology Review Tuberculosis (TB) is a highly communicable disease caused by infection with Mycobacterium tuberculosis. It is one of the most common bacterial infections worldwide and one of the top 10 causes of death (World Health Organization [WHO], 2019). The organism is transmitted via aerosolization (i.e., an airborne route) (Fig. 28.2). When a person with active TB coughs, laughs, sneezes, whistles, or sings, infected respiratory droplets become airborne and may be inhaled by others. Not all TB infections actually develop into active TB (American Lung Association [ALA], 2018). This is because the normal protection of immunity prevents full development of TB in the healthy person (McCance et al., 2019). ( Immunity is the protection from illness or disease that is maintained by the body’s physiologic defense mechanisms. Secondary TB – reactivation of the disease in a previously infected person Etiology M. tuberculosis is a slow-growing, acid-fast rod transmitted via the airborne route. Adults most often infected are those having repeated close contact with an infectious person who has not yet been diagnosed with TB. The risk for infection transmission is reduced after an adult with active TB has received proper drug therapy for 2 to 3 weeks, clinical improvement occurs, and acid-fast bacilli (AFB) in the sputum are reduced. Health Promotion and Maintenance Many adults who acquire TB have risk factors such as homelessness, living in very crowded conditions, or substance use with malnutrition. These risk factors are best managed on a societal level. Communities need to work toward providing adequate housing, substance-use programs that are accessible, and feeding centers or food banks for those in need. On a personal level, many health conditions make it more likely to contract TB if exposed. Adults with these health conditions should avoid people who are ill, stay well nourished, and practice good handwashing and social distancing. Any adult who works with people at high risk of having TB should be screened yearly. History: Assess the patient’s past exposure to TB. Ask about his or her country of origin and travel to or from foreign countries where incidence of TB is high (Benkert & Rayford, 2018). It is important to ask about the results of any previous tests for TB. Also ask whether the patient has had bacille Calmee-Guérin (BCG) vaccine (often given in childhood overseas), which contains attenuated tubercle bacilli. Anyone who has received BCG vaccine within the previous 10 years will have a positive skin test that can complicate interpretation for current TB infection . Usually the size of the skin response decreases each year after BCG vaccination. These patients should be evaluated for TB with a chest x-ray or an interferongamma release assay (IGRA), such as the QuantiFERON-TB Gold test (CDC, 2018e; WHO, 2019). Physical Assessment/Signs and Symptoms The patient with TB has progressive fatigue, lethargy, nausea, anorexia, weight loss, irregular menses, and a low-grade fever. Symptoms may have been present for weeks or months. Night sweats may occur with the fever. A cough with mucopurulent sputum, often streaked with blood, is present. Chest tightness and a dull, aching chest pain occur with the cough. Ask about, assess for, and document the presence of any of these symptoms to help with diagnosis, establish a baseline, and plan nursing interventions Psychosocial Assessment Tuberculosis is a frightening diagnosis. Explain the disease to the patient and family, including the need to maintain good hygiene and avoid infecting others. The patient may feel isolated and shunned. Take time to listen to him or her and help to resolve any concerns. The family and friends of the patient may have similar concerns as well. Often close contacts will be afraid they have contracted the illness. Encourage all close contacts to get tested. Help the patient notify his or her employer, if needed, about required time off. Directly observed therapy may feel threatening. Explain how this helps improve adherence to the long treatment schedule. Diagnostic Assessment Chest x-ray Sputum culture NAAT TST IGRA (e.g., QuantiFERON-TB Gold In-Tube test) TB infection can be tested by methods. In addition to chest x-ray, sputum cultures of blood or respiratory secretions can be tested. Many fully automated nucleic acid amplification tests (NAATs) for TB are used on respiratory secretions. Results of these tests are available in less than 2 hours; however, they have limitations. Tuberculin skin testing (TST), also known as the Mantoux test, is the most commonly used reliable screening test for TB. A small amount (0.1 mL) of purified protein derivative (PPD) is placed intradermally in the forearm. The test is “read” in 48 to 72 hours. An area of induration (localized swelling with hardness of soft tissue), not just redness, measuring 10 mm or greater in diameter, indicates exposure to and possible infection with TB (Fig. 28.3). In adults with reduced immunity , induration of 5 mm is a positive result. If possible, the site is re-evaluated after 72 hours because false-negative readings occur more often after only 48 hours. A positive reaction indicates exposure to TB or the presence of inactive (dormant) disease, not active disease. A reduced skin reaction or a negative skin test does not rule out TB disease or infection of the very old or anyone who has severely reduced immunity . Failure to have a skin response because of reduced immunity when infection is present is called anergy. Blood analysis can be done with interferon-gamma release assays, or IGRAs. The first IGRA was the QuantiFERON-TB Gold In-Tube test. IGRAs show how the patient’s immune system responds to the TB bacterium. A positive result means that the person is infected with TB but does not indicate whether the infection is latent or active. Another blood test, the Xpert MTB/RIF Ultra, which can detect drug-resistant strains of TB and is also recommended for testing people with HIV infection, has been approved by both the CDC and WHO. Sputum culture confirms the diagnosis and is also used to evaluate treatment effectiveness. Enhanced TB cultures take up to 4 weeks for a valid result. After drug therapy is started, sputum samples are obtained at specified intervals. Cultures are usually negative after 3 months of effective treatment. Annual screening is needed for anyone who comes into contact with people who may be infected with TB, including some health care workers. Screening is very important for foreign-born adults and migrant workers. Participation in screening programs is higher when programs are delivered in a culturally sensitive and nonthreatening manner. Urge anyone who is considered high risk to have an annual TB screening test Analysis: Analyze Cues and Prioritize Hypotheses The priority collaborative problems for patients with TB include: 1. Potential for airway obstruction due to thick secretions and weak cough effort 2. Potential for development of drug-resistant disease and spread of infection due to inadequate adherence to therapy regimen 3. Weight loss due to inadequate intake and nausea from therapy regimen 4. Fatigue due to lengthy illness, poor gas exchange, and increased energy demands Planning and Implementation: Generate Solutions and Take Action Promoting Airway Clearance Planning: Expected Outcomes The patient with TB is expected to maintain a patent and adequate airway. Interventions Interventions to maintain a patent airway are similar to those for pneumonia and COPD. Instruct the patient to drink plenty of fluids unless another condition requires restriction. Teach him or her to take a deep breath before coughing. An incentive spirometer may facilitate effective coughing. Reducing Drug-Resistance and Infection Spread Planning: Expected Outcomes The patient with TB is expected to become free of active disease and not spread the disease to others. Improving nutrition, managing fatigue. Many of the interventions for fatigue will be the same as those for improving nutrition. Poor nutrition can lead directly to fatigue Home Care Management Most patients with TB are managed outside the hospital; however, patients may be diagnosed with TB while in the hospital for another problem. Discharge may be delayed if the living situation is high risk or if nonadherence to prescribed drug therapy is likely. Ensure collaboration with other members of the interprofessional team, including the case manager or social service worker in the hospital or the community health nursing agency, to ensure that the patient is discharged to the appropriate environment with continued supervision. Self-Management Education Teach the patient to follow the drug regimen exactly as prescribed and always to have a supply on hand. Teach about side effects and ways of reducing them to promote adherence. Remind him or her that the disease is usually no longer contagious after drugs have been taken for 2 to 3 consecutive weeks and clinical improvement is seen; however, he or she must continue with the prescribed drugs for 6 months or longer as prescribed. Directly observed therapy, in which a health care professional watches the patient swallow the drugs, may be indicated in some situations. This practice leads to more treatment successes, fewer relapses, and less drug resistance. More recently, DOT has been successfully performed using a video format (VDOT) in which patients use a phone or other real-time electronic device to demonstrate compliance with the drug regimen. This method helps patients “live their lives” without having to physically come to a place for DOT. Drawbacks to this method include whether the patient is willing and able to use such a device and how good the connectivity is for both the patient’s device and the nurse’s access to the video (Ingram, 2018). The patient who has weight loss and severe lethargy should gradually resume usual activities. Proper nutrition is needed to prevent infection recurrence. Provide the patient with information about how TB can be spread to others. A key to preventing infection transmission is identifying those in close contact with the infected person so that they can be tested and treated if needed. Multidrug therapy may be indicated to prevent TB in heavily exposed adults or for those who have other health problems that reduce immunity. Health Care Resources Teach the patient to receive follow-up care by a primary health care provider for at least 1 year after active treatment. The American Lung Association (ALA) can provide free information to the patient about the disease and its treatment. In addition, Alcoholics Anonymous (AA) and other health care resources for patients with alcoholism are available if needed. Inform patients who smoke or vape that smoking and vaping further reduce breathing effectiveness. Use the suggestions and interventions discussed in Chapter 24 to help the patient quit or reduce cigaree smoking. Assist the patient who uses illicit drugs to locate a drug-treatment program. Evaluation: Evaluate Outcomes Evaluate the care of the patient with TB based on the identified priority patient problems. The expected outcomes are that he or she: • Effectively clears his or her airways as evidenced by absence of crackles and wheezes on auscultation • Is free of active TB and does not spread the infection • Demonstrates improved nutrition as evidenced by weight maintenance or weight gain • Reports decreased fatigue and increased energy and is able to participate in activities to the extent he or she desires • Returns to his or her pretuberculosis health status. Inhalation Anthrax Pathophysiology Review Inhalation anthrax (respiratory anthrax) is a bacterial infection caused by the gram-positive organism Bacillus anthracis. This organism lives as a spore in soil where grass-eating animals live and graze. Most naturally occurring cases of anthrax are on the skin (cutaneous). Inhalation anthrax accounts for only about 5% of cases and is not spread by person-to-person contact. When infection occurs through the lungs, the disease is nearly 100% fatal without treatment (CDC, 2017). Although inhalation anthrax is an occupational hazard of veterinarians, farmers, and others who frequently contact animal wool, hides, bone meal, and skin, any occurrence in other adults is considered an intentional act of bioterrorism. This organism first forms a spore (i.e., an encapsulated organism that is inactive). When many spores are inhaled deeply into the lungs, they enter white blood cells (WBCs), leave their capsules, and replicate. The active bacteria produce toxins that are released into the infected tissues and into the blood, making the infection worse. Massive edema occurs along with hemorrhage and destruction of lung cells. Infected WBCs spread the organisms rapidly to the lymph nodes and blood, causing bacteremia, sepsis, and meningitis. Lethal toxins produced by the bacteria are the most common cause of death. Inhalation anthrax has two stages: prodromal (or incubation period) and fulminant (with active disease). Symptoms (listed in the Key Features: Inhalation Anthrax box) may take up to 8 weeks to develop after exposure. The prodromal stage is early and difficult to distinguish from influenza or pneumonia. Symptoms include low-grade fever, fatigue, mild chest pain, and a dry, harsh cough. A special feature of inhalation anthrax is that it is not accompanied by upper respiratory symptoms of sore throat or rhinitis. Usually the patient starts to feel beer and symptoms improve in 2 to 4 days. If the diagnosis is made and the patient begins appropriate antibiotic therapy at this stage, the likelihood of survival is high. The fulminant stage begins after the patient feels a lile beer. Usually there is a sudden onset of severe illness, including respiratory distress, hematemesis (bloody vomit), dyspnea, diaphoresis, stridor, chest pain, and cyanosis. High fever, hemorrhagic mediastinitis, and pleural effusions develop. As the infection spreads through the blood, septic shock and hemorrhagic meningitis develop. Death often occurs within 24 to 36 hours even if antibiotics are started in this stage. Endemic/Geographic Respiratory Infection Any organism can cause a pulmonary infection if the exposure is high enough, if the adult has lile or no acquired immunity to it, or if the adult’s general immune responses are reduced by age, drugs, or other health problems. A variety of respiratory infections are endemic , meaning that the causative organism is much more common within a geographic location, but even then the incidence of the infection is relatively low. Adults living in these areas have often developed some immunity to the organism over time and usually only develop the infection if they come into contact with large numbers of the organism or have a severely reduced immune response. Most commonly, the organisms are sporeforming fungi, although some viral infections also have endemic tendencies. Table 28.4 lists common endemic respiratory infections in North America. These organisms are part of the environment. Healthy adults in endemic areas who are most susceptible to infection are those who have intense exposures. For soil-borne organisms, adults who dig in the soil, farm, or work in construction in which soil is disturbed can be heavily exposed. Everyone can be exposed when the soil is disturbed by dust storms, tornados, flooding, and other types of natural disasters. Working and living in buildings in which demolition and/or reconstruction are occurring can release spores trapped within walls that then become airborne. Working with or camping in areas with organisms that live in soil or decomposing wood and leaves can result in significant exposures. All of these respiratory infections resemble influenza or pneumonia with fever, cough, headache, muscle aches, chest pain, and night sweats and are often misdiagnosed. Also, these infections are not contagious from person to person. Identification of the specific organism is important for specific treatment and prevention of complications. Always ask anyone with respiratory infection symptoms whether they have visited endemic regions to help identify possible sources, expected courses, and appropriate management strategies. Depending on the health and immunity of the infected person and the number of spores present in the respiratory tract, the resulting infection can be mild, moderate, severe, or widely disseminated to other major organs. Special populations, such as older adults, pregnant women, and others who are immunocompromised are at greater risk for more severe disease. With fungal infections, a chronic infection state is possible. Fungal infections are difficult to eradicate. With moderate severity, oral antifungal agents may be prescribed for weeks to months. For more severe disease, IV g y p antifungal agents, including amphotericin, may be needed initially and followed by long-term oral agents. Supportive care is similar to that provided for patients with influenza and pneumonia. Chapter 33: Care of Patients with Vascular Problems Perfusion Concept Exemplar: Hypertension Desired blood pressure For people over 60 Below 150/90 For people younger than 60 According to Joint National Committee 8 (JNC 8) guidelines, patients whose blood pressures are above these goals should be treated with drug therapy and lifestyle modifications Below 140/90 HTN most common health problem in primary care settings & can cause stroke, MI, kidney failure, and death if not treated effectively. AHA: bp below 130/80 Table 1: AHA/ACCa Guideline Recommendations by Blood Pressure Category Mechanisms that Influence Blood Pressure Consequently, any factor that increases PVR (peripheral vascular resistance), HR, or SV increases the systemic arterial pressure. Conversely, any factor that decreases PVR, HR, or SV decreases the systemic arterial pressure and can cause decreased perfusion to body tissues. control systems that maintain blood pressure Four control systems play a major role in maintaining blood pressure: The arterial baroreceptor system: primarily in the carotid sinus, aorta, and wall of the left ventricle. They monitor the level of arterial pressure and counteract a rise in arterial pressure through vagally mediated cardiac slowing and vasodilation with decreased sympathetic tone. Reflex control of circulation therefore elevates the systemic arterial pressure when it falls and lowers it when it rises. Why baroreceptor control fails in hypertension is not clear Regulation of body fluid volume: if there is an excess of sodium and/or water in a person’s body, the BP rises through complex physiologic mechanisms that change the venous return to the heart, producing a rise in cardiac output (CO). If the kidneys are functioning adequately, a rise in systemic arterial pressure produces diuresis (excessive voiding) and a fall in pressure. Pathologic conditions change the pressure threshold at which the kidneys excrete sodium and water, thereby altering the systemic arterial pressure. The renin-angiotensin-aldosterone system: kidney produces renin, an enzyme that acts on angiotensinogen to split off angiotensin I, which is converted by an enzyme in the lung to form angiotensin II. Angiotensin II has strong vasoconstrictor action on blood vessels and is the controlling mechanism for aldosterone release. Aldosterone then works on the collecting tubules in the kidneys to reabsorb sodium. Sodium retention inhibits fluid loss, thus increasing blood volume and subsequent BP. Renin acts on angiotensin angiotensin I angiotensin II angiotensin II acts on blood vessels aldosterone release, aldosterone reabsorbs sodium = fluid loss prevented, blood volume increased bp increased Inappropriate secretion of renin may cause increased peripheral vascular resistance (PVR) in patients with hypertension. When the BP is high, renin levels should decrease because the increased renal arteriolar pressure usually inhibits renin secretion. However, for most people with essential hypertension, renin levels remain normal Vascular autoregulation: the process of vascular autoregulation, which keeps perfusion of tissues in the body relatively constant, appears to be important in causing hypertension. However, the exact mechanism of how this system works is poorly understood. Essential Hypertension Most common type of HTN Results in damage to vital organs Causes medial hyperplasia (thickening) of arterioles: As the blood vessels thicken and perfusion decreases, body organs are damaged. These changes can result in myocardial infarctions (MIs), strokes, peripheral vascular disease (PVD), or kidney failure Common risk factors: Obesity Smoking Stress Family history Secondary Hypertension: Caused by specific disease states or drugs Hypertensive Crisis (Malignant Hypertension) Medical emergency Symptoms include morning headaches, blurred vision, dyspnea, uremia Diastolic may be > 150 mm Hg Systolic may be > 200 mm Hg *uremia (accumulation in the blood of substances ordinarily eliminated in the urine A person with this health problem usually has symptoms such as morning headaches, blurred vision, and dyspnea and/or symptoms of uremia (accumulation in the blood of substances ordinarily eliminated in the urine). Patients are often in their 30s, 40s, or 50s with their systolic BP greater than 200 mm Hg. The diastolic BP is greater than 150 mm Hg or greater than 130 mm Hg when there are pre-existing complications. Unless intervention occurs promptly, a patient with hypertensive crisis may experience kidney failure, left ventricular heart failure, or stroke. Hypertension: Etiology and Genetic Risk Essential: African American ethnicity Hyperlipidemia Smoking Older than 60 years old Obesity, physical inactivity Secondary: kidney disease *most common cause of secondary HTN Hypertension can develop when there is any sudden damage to the kidneys. Renovascular hypertension is associated with narrowing of one or more of the main arteries carrying blood directly to the kidneys, known as renal artery stenosis (RAS). Many patients have been able to reduce the use of their antihypertensive drugs when the narrowed arteries are dilated through angioplasty with stent placement. Hypertension: Health Promotion and Maintenance Weight: weight reduction Dash: Dash diet diet that is high in fruits, vegetables, and low-fat dairy products; enhance intake of potassium, calcium, magnesium, and fiber. Reduce: reduce intake of dietary sodium Reduce the intake of dietary sodium. The optimal goal is less than 1500 mg of sodium per day. Increase: increase physical activity includes aerobic exercise, resistance training, and static isometric exercise. Hypertension: Assessment: Recognize Cues History: Risk factors Kidney or CVD Drug therapy or illicit drug use Collect data on the patient’s age; ethnic origin or race; family history of hypertension; average dietary intake of calories, sodium- and potassium-containing foods and alcohol; and exercise habits. Physical assessment/Signs & symptoms May have no symptoms Fundoscopic examination: Funduscopic examination of the eyes to observe vascular changes in the retina is done by a skilled health care practitioner. The appearance of the retina can be a reliable index of the severity and prognosis of hypertension. Physical assessment is also helpful in diagnosing several conditions that produce secondary hypertension. Abdominal bruits: The presence of abdominal bruits is typical of patients with renal artery stenosis (RAS). Tachycardia, sweating, and pallor may suggest a pheochromocytoma (adrenal medulla tumor). Coarctation of the aorta is evidenced by elevation of blood pressure in the arms, with normal or low blood pressure in the lower extremities. Psychosocial assessment Hypertension: Assessment: Recognize Cues Diagnostic assessment Urinalysis for protein, RBC, BUN, creatinine Chest x-ray (cardiomegaly) ECG shows degree of cardiac involvement Although no laboratory tests are diagnostic of essential hypertension, several laboratory tests can assess possible causes of secondary hypertension. Kidney disease can be diagnosed by the presence of protein and red blood cells in the urine, elevated levels of blood urea nitrogen (BUN), and elevated serum creatinine levels. The creatinine clearance test directly indicates the glomerular filtration ability of the kidneys. The normal value is 107 to 139 mL/min for men and 87 to 107 mL/min for women (Pagana et al., 2018). Decreased levels indicate acute or chronic kidney disease. Urinary test results are positive for the presence of catecholamines in patients with a pheochromocytoma (tumor of the adrenal medulla). An elevation in levels of serum corticoids and 17-ketosteroids in the urine is diagnostic of Cushing disease. No specific x-ray studies can diagnose hypertension. Routine chest radiography may help recognize cardiomegaly (heart enlargement). An electrocardiogram (ECG) determines the degree of cardiac involvement. Left atrial and ventricular hypertrophy is the first ECG sign of heart disease resulting from hypertension. Left ventricular remodeling can be detected on the 12-lead ECG Hypertension: Analysis: Analyze Cues and Prioritize Hypotheses The priority collaborative problems for most patients with hypertension are: Need for health teaching due to the plan of care for hypertension management Potential for decreased adherence due to side effects of drug therapy and necessary changes in lifestyle Hypertension: Planning and Implementation: Generate Solutions and Take Action Health teaching Lifestyle changes are considered the foundation of hypertension control. If these changes are unsuccessful, the primary care provider considers the use of antihypertensive drugs. There is no surgical treatment for essential hypertension. However, surgery may be indicated for certain causes of secondary hypertension, such as kidney disease, coarctation of the aorta, and pheochromocytoma. Lifestyle modifications as in Health promotion Abstain or decrease alcohol consumption (no more than one drink a day for women and two drinks a day for men), Stop smoking and tobacco use, Use relaxation techniques to reduce stress • Restrict dietary sodium according to ACC/AHA guidelines • Reduce weight, if overweight or obese • Implement a heart-healthy diet, such as the DASH diet • Increase physical activity with a structured exercise program • Abstain or decrease alcohol consumption (no more than one drink a day for women and two drinks a day for men) • Stop smoking and tobacco use • Use relaxation techniques to reduce stress Drug therapy: Beta-adrenergic blockers, Renin inhibitors, Central alpha agonists, Alphaadrenergic agonists, Diuretics, Calcium channel blockers, ACE inhibitors, Angiotensin II receptor antagonists, Aldosterone receptor antagonists In the largest hypertensive trial done to date, Antihypertensive and LipidLowering Treatment to Prevent Heart Attack Trial (ALLHAT), the use of diuretics has been practically unmatched in preventing the cardiovascular complications of HTN Current guidelines recommend use of one or more of these four classes of drugs: thiazide-type diuretics, calcium channel blockers (CCBs), angiotensinconverting enzyme inhibitors (ACEIs), and angiotensin II receptor blockers (ARBs). Using an ACEI, ARB, and/or renin inhibitor simultaneously is potentially harmful and is not recommended in the treatment of hypertension. Patients who do not respond to these first-line drugs may be placed on other diuretics, an aldosterone receptor antagonist (blocker), a beta-adrenergic blocker, or a renin inhibitor. Diuretics. Diuretics are the first type of drugs for managing hypertension. Three basic types of diuretics are used to decrease blood volume and lower blood pressure in the order of how commonly they are typically prescribed: • Thiazide (low-ceiling) diuretics, such as hydrochlorothiazide, inhibit sodium, chloride, and water reabsorption in the distal tubules while promoting potassium, bicarbonate, and magnesium excretion. However, they decrease calcium excretion, which helps prevent kidney stones and bone loss. Because of the low cost and high effectiveness of thiazide-type diuretics, they are usually the drugs of choice for patients with uncomplicated hypertension. These drugs can be prescribed as a single agent or in combination with other classes of drugs. Loop (high-ceiling) diuretics, such as furosemide and torsemide, inhibit sodium, chloride, and water reabsorption in the ascending loop of Henle and promote potassium excretion Complementary and Integrative Health Garlic, coenzyme Q10, and fish oil: Evidence by consensus and case reports do support garlic’s cholesterol-lowering ability and its ability to decrease blood pressure in patients with hypertension (National Center for Complementary and Integrative Health, 2018). Garlic can increase the risk of bleeding in patients taking anticoagulants and can interfere with the effectiveness of some drugs. Teach patients to check with their primary health care provider before starting any herbal therapy because of possible side effects and interactions with other herbs, foods, or drugs. Some patients have also had success with biofeedback, meditation, and acupuncture as part of their overall management plan. These methods may be most useful as adjuncts for patients who experience continuous and severe stress. Promoting adherence to the plan of care Planning: Expected Outcomes The patient with hypertension is expected to adhere to the plan of care, including making necessary lifestyle changes. Interventions Patients who require medications to control essential hypertension usually need to take them for the rest of their lives. Some patients stop taking them because they have no symptoms and have troublesome side effects. In the hospital setting, interprofessional collaboration with the pharmacist to discuss the outcomes of therapy with the patient, including potential side effects, can help the patient tailor the therapeutic regimen to his or her lifestyle and daily schedule. Patients who do not adhere to antihypertensive treatment are at a high risk for target organ damage and hypertensive crisis, a severe elevation in blood pressure (>180/120) that can cause organ damage in the kidneys or heart (target organs) (see the Best Practice for Patient Safety & Quality Care: Emergency Care of Patients With Hypertensive Crisis box). Patients in hypertensive crisis are admitted to critical care units, where they receive IV antihypertensive therapy such as nitroprusside, nicardipine, fenoldopam, or labetalol. For adults without a compelling condition, systolic BP should be reduced by no more than 25% within the first hour; then, if stable, to 160/100 mm Hg within the next 2 to 6 hours; and then cautiously to normal during the following 24 to 48 hours (Whelton et al., 2017). A gradual reduction in blood pressure is preferred because rapid reduction can cause cerebral ischemia, MI, and renal failure. Provide oxygen to the patient and monitor oxygen saturation levels. When the patient’s blood pressure stabilizes, oral antihypertensive drugs are given. Hypertension: Care Coordination and Transition Management Home care management: Hypertension is a chronic illness. Allow patients to verbalize feelings about the disease and its treatment. Emphasize that their involvement in the collaborative plan of care can lead to control of the disease and can prevent complications. Some patients do not adhere to their drug therapy regimen at home because they have no symptoms or they simply forget to take their drugs. Others may think they are not sick enough to need medication. Some patients may assume that once their blood pressure (BP) returns to normal levels, they no longer need treatment. They may also stop taking their drugs because of side effects or cost. Develop a plan with the patient and family and identify ways to encourage adherence to the plan of care. Self management education: Health teaching is essential to help patients become successful in managing their BP. Provide oral and written n information about the indications, dosage, times of administration, side effects, and drug interactions for antihypertensives. Stress that medication must be taken as prescribed; when all of it has been consumed, the prescription must be renewed on a continual basis. Suddenly stopping drugs such as beta blockers can result in angina (chest pain), myocardial infarction (MI), or rebound hypertension. Urge patients to report unpleasant side effects such as excessive fatigue, cough, or sexual dysfunction. In many instances, an alternative drug can be prescribed to minimize certain side effects. Teach the patient to obtain an ambulatory BP monitoring (ABPM) device for use at home so the pressure can be checked. Evaluate the patient’s and family’s ability to use this device. If weight reduction is a desired outcome, suggest having a scale in the home for weight monitoring. For patients who do not want to self-monitor, are not able to self-monitor, or have “white-coat” syndrome when they go to their primary health care provider (causing elevated BP), continuous ABPM may be used. The monitor is worn for 24 hours or longer while patients perform their normal daily activities. BP is automatically taken every 15 to 30 minutes and recorded for review later. The advantage of this technique is that the primary health care provider can view the changes in BP readings throughout the 24-hour period to get a picture of a true BP value. Research strongly supports 24-hour ambulatory BP monitoring as a first-line procedure to determine the need for antihypertensive therapy (U.S. Preventive Services Task Force, 2017). Instruct the patient about sodium restriction, weight maintenance or reduction, alcohol restriction, stress management, and exercise. If necessary, also explain about the need to stop using tobacco, especially smoking. Health care resources A home care nurse may be needed for follow-up to monitor the BP. Evaluate patient or family ability to obtain accurate BP measurements and assess adherence with treatment. The American Heart Association, the Red Cross, or a local pharmacy may be used for free BP checks if patients cannot buy equipment to monitor their BP. Health fairs and BP screening programs located in faith-based centers are also available in most locations. Hypertension: Evaluation: Evaluate Outcomes Verbalize understanding of the plan of care, including drug therapy and any necessary lifestyle changes Report adverse drug effects, such as coughing, dizziness, or sexual dysfunction, to the primary health care provider immediately Consistently adhere to the plan of care, including regular follow-up with the primary health care provider Arteriosclerosis and Atherosclerosis Pathophysiology Overview Arteriosclerosis Thickening or hardening of arterial wall Often associated with aging Atherosclerosis Type of arteriosclerosis involving formation of plaque within arterial wall Leading risk factor for cardiovascular disease Arteriosclerosis is a thickening, or hardening, of the arterial wall that is often associated with aging. Atherosclerosis, a type of arteriosclerosis, involves the formation of plaque within the arterial wall and is the leading risk factor for cardiovascular disease (CVD). Usually the disease affects the larger arteries, such as coronary artery beds; aorta; carotid and vertebral arteries; renal, iliac, and femoral arteries; or any combination of these. The exact pathophysiology of atherosclerosis is not known, but the condition is thought to occur from blood vessel damage that causes inflammation (Fig. 33.2). Inflammation occurs in response to cellular injury. After the vessel becomes inflamed, a fatty streak appears on the intimal surface (inner lining) of the artery. Through the process of cellular proliferation, collagen migrates over the fatty streak, forming a fibrous plaque. The fibrous plaque is often elevated and protrudes into the vessel lumen, partially or completely obstructing blood flow through the artery. Plaques are either stable or unstable. Unstable plaques are prone to rupture and are often clinically silent until they rupture. In the final stage, the fibrous plaques become calcified, hemorrhagic, ulcerated, or thrombosed and affect all layers of the vessel. The rate of progression of the process may be influenced by genetic factors; certain chronic diseases (e.g., diabetes mellitus); and lifestyle habits, including smoking, eating habits, and level of exercise. When stable plaque ruptures, thrombosis (blood clot) and constriction obstruct the vessel lumen, causing inadequate perfusion and oxygenation to distal tissues. Unstable plaque rupture causes more severe damage. After the rupture occurs, the exposed underlying tissue causes platelet adhesion and rapid thrombus formation. The thrombus may suddenly block a blood vessel, resulting in ischemia and infarction (e.g., myocardial infarction [MI]). Endothelial (intimal) injury of the major arteries of the body can be caused by many factors. Elevated levels of lipids (fats) such as low-density lipoprotein cholesterol (LDL-C) and decreased levels of high-density lipoprotein cholesterol (HDL-C) can cause chemical injuries to the vessel wall. (Chapter 30 discusses lipids in detail.) Chemical injury can also be caused by elevated levels of toxins in the bloodstream, which may occur with renal failure or by carbon monoxide circulating in the bloodstream from cigarette smoking. The vessel wall can be weakened by the natural process of aging or by diseases such as hypertension. Genetic predisposition and diabetes have a major effect on the development of atherosclerosis. Some patients have familial hyperlipidemia, an elevation of serum lipid levels. In these people, the liver makes excessive cholesterol and other fats. However, some people with hereditary atherosclerosis have a normal blood cholesterol level. The reason for the development and progression of plaque in these patients is not understood (McCance & Huether, 2019). Adult patients of any age with severe diabetes mellitus frequently have premature and severe atherosclerosis from microvascular damage. The premature atherosclerosis occurs because diabetes promotes an increase in LDL-C and triglycerides (TGs) (lipids) in plasma. In addition, arterial damage may result from the effect of hyperglycemia. Other factors are indirectly related to atherosclerosis development. A list of risk factors is found in Table 33.4. Pathophysiology of Atherosclerosis Discuss physical manifestations: Monitor BP Palpate pulses in all major sites of body Assess for prolonged capillary refill Assess for bruit Atherosclerosis The image to the left compares a normal artery to an artery with fat buildup (plaque) and an artery blocked with fat Arteriosclerosis and Atherosclerosis: Assessment: Noticing Assess blood pressure in both arms Palpate carotid arteries separately Capillary refill Bruits Cholesterol and triglycerides The assessment of a patient with atherosclerosis includes a complete cardiovascular assessment because associated heart disease is often present. Because of the high incidence of hypertension in patients with atherosclerosis, assess the blood pressure in both arms. Palpate pulses at all the major sites on the body and note any differences. Palpate each carotid artery separately to prevent blocking blood flow to the brain! Also feel for temperature differences in the lower extremities and check capillary filling. Prolonged capillary filling (>3 seconds in youngto–middle-age adults; >5 seconds in older adults) generally indicates poor circulation, although this is not the most reliable indicator of perfusion. With severe atherosclerotic disease, the extremity may be cool or cold with a diminished or absent pulse. Many patients with vascular disease have a bruit in the larger arteries, which can be heard with a stethoscope or Doppler probe. A bruit is a turbulent, swishing sound, which can be soft or loud in pitch. It is heard as a result of blood trying to pass through a narrowed artery. A bruit is considered abnormal, but it does not indicate the severity of disease. Bruits often occur in the carotid, aortic, femoral, and popliteal arteries. Patients with atherosclerosis often have elevated lipids, including cholesterol and triglycerides (TGs). Elevated cholesterol levels are confirmed by HDL and LDL measurements. Increased low-density lipoprotein cholesterol (LDL-C) (“bad” cholesterol) levels and low highdensity lipoprotein cholesterol (HDL-C) (“good” cholesterol) indicate that a person is at an increased risk for atherosclerosis. The triglyceride level may also be elevated with atherosclerosis. Elevated TGs are considered a marker for other lipoproteins. They also suggest metabolic syndrome, which increases the risk for coronary disease (see Chapter 30 for in-depth serum lipid information). Arteriosclerosis and Atherosclerosis: Interventions: Take Action Low risk people should have total serum cholesterol levels evaluated every 4-6 years Those with risk factors and older than 40 years old should have evaluation more frequently Atherosclerosis progresses for years before signs and symptoms occur. Adults who are at risk for the disease can often be identified through cholesterol screening and history. Because of the high incidence in the United States, low-risk people 20 years of age and older are advised to have their total serum cholesterol level evaluated at least once every 4 to 6 years. More frequent measurements are suggested for people with multiple risk factors and those older than 40 years. People with multiple risk factors are grouped into high-risk patient categories. Interventions for patients with atherosclerosis or those at high risk for the disease focus on lifestyle changes. Teach patients about the need to make daily changes by avoiding or minimizing modifiable risk factors. Modifiable risk factors are those that can be changed or controlled by the patient, such as smoking, weight management, and exercise. Nutrition is one of the most important parts of the risk-reduction plan. If lipoprotein levels do not improve after lifestyle changes, the primary health care provider may prescribe drug therapy to lower cholesterol and/or TGs. Modifiable risk factors Nutrition Consume a dietary pattern that emphasizes intake of vegetables, fruits, whole grains Consumer low-fat diary products, poultry (without the skin), fish, legumes, nontropical (e.g., canola) vegetable oils, and nuts. • Limit intake of sweets, sugar-sweetened beverages, and red meats. • Aim for a dietary pattern that includes 5-6% of calories from saturated fat; limiting trans fat Activity These guidelines are similar to the Dietary Approaches to Stop Hypertension (DASH), which also recommend daily sodium, potassium, and fiber amounts (National Heart, Lung, and Blood Institute, 2018). Interprofessional collaboration with the registered dietitian nutritionist to teach the patient about the types of fat content in food is encouraged. Meats and eggs contain mostly saturated fats and are high in cholesterol. Instruct patients about increasing dietary fiber to 30 g each day, which is consistent with DASH guidelines. aerobic physical activity three or four times a week to reduce LDL-C levels. Each session should last for 40 minutes on average and involve moderate-tovigorous physical activity ( Drug therapy For patients with elevated total and LDL-C levels that do not respond adequately to dietary intervention, the primary health care provider prescribes a cholesterol-lowering agent. Drug choice and dosing depend on the serum cholesterol level, the degree to which the level needs to be decreased, and the patient’s age (Grundy et al., 2018). Because most of these drugs can produce side effects, they are generally given only when nonpharmacologic management has been unsuccessful. A class of drugs known as 3-hydroxy-3-methylglutaryl coenzyme A (HMGCoA) reductase inhibitors (statins) successfully reduces total cholesterol in most patients when used for an extended period. Examples include lovastatin, simvastatin, and pitavastatin, which lower both LDL-C and triglyceride levels (Table 33.5). Drug Therapy HMG-CoA reductase inhibitors (statins) Ezetimibe: A different type of lipid-lowering agent, ezetimibe, may be used in place of or in combination with statin-type drugs. This drug inhibits the absorption of cholesterol through the small intestine, leading to a decrease in the delivery of intestinal cholesterol to the liver, and increases the clearance of cholesterol from the blood. Combination drugs (e.g., ezetimibe with simvastatin): Vytorin is a combination drug containing ezetimibe and simvastatin. This drug works two ways—by reducing the absorption of cholesterol and by decreasing the amount of cholesterol synthesis in the liver. Amlodipine and atorvastatin are combined as Caduet to decrease blood pressure while decreasing triglycerides (TGs), increasing HDL, and lowering LDL. Combining drugs may improve adherence for the patient who is often taking multiple drugs. PCSK9 inhibitors: The U.S. Food and Drug Administration (FDA) approved the drug class PCSK9 inhibitors for use in patients with familial hypercholesterolemia or for those in whom existing therapies are unable to reduce LDLs. These potent drugs inhibit PCSK9, which is a protease produced primarily in the liver that can cause elevations in LDLs. Alirocumab and evolocumab are administered by subcutaneous injection on a monthly or bimonthly basis for patients who are on maximally tolerated doses of statins. Peripheral Arterial Disease (PAD) Pathophysiology Overview Peripheral vascular disease Systemic atherosclerosis Alters natural flow of blood through arteries and veins of peripheral circulation Result of systemic atherosclerosis Lower Extremity Arterial Disease The image to the left shows common locations of inflow and outflow lesions. Peripheral Arterial Disease (PAD): Assessment: Recognize Cues Stage I: Asymptomatic Stage II: Claudication Stage III: Rest Pain Stage IV: Necrosis/Gangren e Peripheral vascular disease (PVD) includes disorders that change the natural flow of blood through the arteries and veins of the peripheral circulation, causing decreased perfusion to body tissues. It affects the legs much more frequently than the arms. In general, a diagnosis of PVD implies arterial disease (peripheral arterial disease [PAD]) rather than venous involvement. Some patients have both arterial and venous disease. The cost of the disease is very high and is expected to increase as “baby boomers” age and obesity in the United States continues to be a major health problem. PAD is a result of systemic atherosclerosis. It is a chronic condition in which partial or total arterial occlusion (blockage) decreases perfusion to the extremities. The tissues below the narrowed or obstructed arteries cannot live without an adequate oxygen and nutrient supply. PAD in the legs is sometimes referred to as lower extremity arterial disease (LEAD). Obstructions are classified as inflow or outflow, according to the arteries involved and their relationship to the inguinal ligament (Fig. 33.3). Inflow obstructions involve the distal end of the aorta and the common, internal, and external iliac arteries. They are located above the inguinal ligament. Outflow obstructions involve the femoral, popliteal, and tibial arteries and are below the superficial femoral artery (SFA). Gradual inflow occlusions may not cause significant tissue damage. Gradual outflow occlusions typically do. Atherosclerosis is the most common cause of chronic arterial obstruction; therefore the risk factors for atherosclerosis apply to PAD as well (see Table 33.4). Advancing age also increases the risk for disease related to atherosclerosis. Patients with PAD have an increased risk for developing chronic angina, MI, or stroke. PAD is a marker for systemic atherosclerotic disease, making people with PAD more likely to have atherosclerosis in other vascular beds such as the coronary, carotid, and renal arteries and the abdominal aorta (Benjamin et al., 2018). About 8.5 million people in the United States age 40 and older have PAD. African Americans are affected more often than any other group, most likely because they have many risk factors such as diabetes and hypertension (Benjamin et al., 2018 Peripheral Arterial Disease (PAD): Assessment: Recognize Cues Hair loss & dry, scaly, pale or mottled skin, thickened toenails Severe arterial disease Extremity is cold with gray-blue or darkened. Pall may occur with extremity elevation. Dependent rubor. Muscle atrophy The clinical course of chronic PAD can be divided into four stages (see the Key Features: Chronic Peripheral Arterial Disease box). Patients do not experience symptoms in the early stages of disease. Many patients are not diagnosed until they develop leg pain. Most patients initially seek medical attention for a classic leg pain known as intermittent claudication (a term derived from a word meaning “to limp”). Usually they can walk only a certain distance before discomfort, such as cramping or burning muscular pain, forces them to stop. The pain stops with rest. When patients resume walking, they can walk the same distance before it returns. Thus the pain is considered reproducible. As the disease progresses, they can walk only shorter and shorter distances before pain recurs. Ultimately it may occur even while at rest. Rest pain, which may begin while the disease is still in the stage of intermittent claudication, is a numb or burning sensation, often described as feeling like a toothache that is severe enough to awaken patients at night. It is usually located in the toes, the foot arches, the forefeet, the heels, and rarely in the calves or ankles. Patients can sometimes alleviate pain by keeping the limb in a dependent position (below the heart). Those with rest pain often have advanced disease that may result in limb loss. Patients with inflow disease have discomfort in the lower back, buttocks, or thighs. Patients with mild inflow disease have discomfort after walking about two blocks. This discomfort is not severe but causes them to stop walking. It is relieved with rest. Patients with moderate inflow disease experience pain in these areas after walking about one or two blocks. The discomfort is described as being more like pain, but it eases with rest most of the time. Severe inflow disease causes severe pain after walking less than one block. These patients usually have rest pain. Patients with outflow disease describe burning or cramping in the calves, ankles, feet, and toes. Instep or foot discomfort indicates an obstruction below the popliteal artery. Those with mild outflow disease experience discomfort after walking about five blocks. Rest relieves this discomfort. Patients with moderate outflow disease have pain after walking about two blocks. Intermittent rest pain may be present. Those with severe outflow disease usually cannot walk more than one-half block. They may hang their feet off the bed at night for comfort & report more frequent rest pain than patients with inflow disease. Specific findings for PAD depend on the severity of the disease. Observe for loss of hair on the lower calf, ankle, and foot; dry, scaly, dusky, pale, or mottled skin; and thickened toenails. With severe arterial disease, the extremity is cold and gray-blue (cyanotic) or darkened. Pallor may occur when the extremity is elevated. Dependent rubor (redness) may occur when the extremity is lowered (Fig. 33.4). Muscle atrophy can result from prolonged chronic arterial disease Palpate all pulses in both legs. The most sensitive and specific indicator of arterial function is the quality of the posterior tibial pulse because the pedal pulse is not palpable in a small percentage of people. The strength of each pulse should be compared bilaterally. Note early signs of ulcer formation or complete ulcer formation, a complication of PAD. Arterial and venous stasis ulcers differ from diabetic ulcers (see the Key Features: Chronic Peripheral Arterial Disease box). Initially, arterial ulcers are painful and develop on the toes (often the great toe), between the toes, or on the upper aspect of the foot. With prolonged occlusion, the toes can become gangrenous. Typically, the ulcer is small and round with a hollow appearance and well-defined borders. Peripheral Arterial Disease (PAD): Diagnostic Assessment MRA Segmental systolic blood pressure measurement Plethysmography Ankle-brachial index (ABI) The ABI is calculated by dividing the ankle systolic BPs by the higher of the left and right brachial systolic BP. Exercise tolerance training A normal ABI is 0.91 to 1.30 and indicates adequate BP in the extremities. An ABI between 0.71 and 0.90 indicates mild PAD, between 0.41 and 0.70 indicates moderate PAD, and <0.40 indicates severe PAD. Magnetic resonance angiography (MRA) is commonly used to assess blood flow in the peripheral arteries. A contrast medium is used to help visualize blood flow through these arteries. This test is often the only one used to diagnose PAD, although computed tomography angiography (CTA) may also be performed. Using a Doppler probe, segmental systolic blood pressure measurements of the lower extremities at the thigh, calf, and ankle are an inexpensive, noninvasive method of assessing PAD. Normally, blood pressure readings in the thigh and calf are higher than those in the upper extremities. With the presence of arterial disease, these pressures are lower than the brachial pressure. With inflow disease, pressures taken at the thigh level indicate the severity of disease. Mild inflow disease may cause a difference of only 10 to 30 mm Hg in pressure on the affected side compared with the brachial pressure. Severe inflow disease can cause a pressure difference of more than 40 to 50 mm Hg. The ankle pressure is normally equal to or more than the brachial pressure. To evaluate outflow disease, compare ankle pressure with the brachial pressure, which provides a ratio known as the ankle-brachial index (ABI). The value can be derived by dividing the ankle blood pressure by the brachial blood pressure. An ABI of less than 0.90 in either leg is diagnostic of PAD. Patients with diabetes are known to have a falsely elevated ABI. Peripheral Arterial Disease (PAD) Interventions: Take Action Collaborative management of PAD may include nonsurgical interventions and/or surgery. The patient must first be assessed to determine if the altered tissue perfusion is caused by arterial disease, venous disease, or both. Exercise, positioning, promoting vasodilation, drug therapy, and invasive nonsurgical procedures are used to increase arterial flow to the affected leg(s). Exercise & positioning Promoting vasodilation Exercise may improve arterial blood flow to the affected leg through buildup of the collateral circulation. Collateral circulation provides blood to the affected area through smaller vessels that develop and compensate for the occluded vessels. Exercise is individualized for each patient, but people with severe rest pain, venous ulcers, or gangrene should not participate. Others with PAD can benefit from exercise that is started gradually and slowly increased. Instruct the patient to walk until the point of claudication, stop and rest, and then walk a little farther. Eventually, he or she can walk longer distance as collateral circulation develops. Collaborate with the primary health care provider and physical therapist in determining an appropriate exercise program. Exercise rehabilitation has been used to relieve symptoms but requires a motivated patient. The cost of supervised sessions generally is not reimbursed by health care insurance. Positioning to promote circulation has been somewhat controversial. Some patients have swelling in their extremities. Teach them to avoid raising their legs above the heart level because extreme elevation slows arterial blood flow to the feet. Vasodilation can be achieved by providing warmth to the affected extremity and preventing long periods of exposure to cold. Encourage the patient to maintain a warm environment at home and to wear socks or insulated shoes at all times. Caution the patient to avoid the application of direct heat to the limb with heating pads or extremely hot water. Sensitivity is decreased in the affected limb. Burns may result. Encourage patients to prevent exposure of the affected limb to the cold because cold temperatures cause vasoconstriction (decreasing of the diameter of the blood vessels) and therefore decrease arterial perfusion. Emotional stress, caffeine, and nicotine also can cause vasoconstriction. Emphasize that complete abstinence from smoking or chewing tobacco is essential to prevent vasoconstriction. The vasoconstrictive effects of each cigarette may last up to 1 hour after the cigarette is smoked. Drug therapy (anticoagulation or antiplatelet agents) For patients with chronic PAD, prescribed drugs include hemorheologic and antiplatelet agents. Pentoxifylline is a hemorheologic agent that increases the flexibility of red blood cells. It decreases blood viscosity by inhibiting platelet aggregation and decreasing fibrinogen and thus increases blood flow in the extremities. Many patients report limited improvement in their daily lives after taking pentoxifylline, and evidence indicates minimal benefit. However, those with extremely limited endurance for walking have reported improvement to the point that they can perform some activities (e.g., walk to the mailbox or dining room) that were previously impossible. Antiplatelet agents, such as aspirin and clopidogrel, are commonly used. If there are no contraindications to antiplatelet therapy, patients with symptomatic PAD should receive aspirin or clopidogrel to reduce MI, stroke, and vascular death (Gerhard-Herman et al., 2017). Some patients receive both drugs (dual antiplatelet therapy). Evidence suggests that dual antiplatelet therapy is reasonable to reduce the risk of limb-related events in patients with symptomatic PAD after lower extremity revascularization (GerhardHerman et al., 2017). Patients who are taking clopidogrel should not eat grapefruit or drink grapefruit juice because of risk of kidney failure, GI bleeding, heart failure, or even death. Patients who experience disabling intermittent claudication may also benefit from phosphodiesterase inhibitors such as cilostazol because it can help improve symptoms and increasing walking distance. This drug can also increase HDL-C levels. Teach patients taking the drug that it may cause headaches and GI disturbances, especially flatulence (gas) and diarrhea. Controlling hypertension can improve tissue perfusion by maintaining pressures that are adequate to perfuse the periphery but not constrict the vessels. Teach about the effect of blood pressure on the circulation and instruct in methods of control. For example, patients taking beta blockers may have drug-related claudication or a worsening of symptoms. The primary health care provider closely monitors those who are receiving beta blockers. If the patient has high serum lipids, lipid-lowering drugs such as statins are used Percutaneous vascular intervention A nonsurgical but invasive approach for improving arterial flow is the use of percutaneous vascular intervention. This procedure requires an arterial puncture in the patient’s groin. One or more arteries are dilated with a balloon catheter advanced through a cannula, which is inserted into or above an occluded or stenosed artery. When the procedure is successful, it opens the vessel and improves arterial blood flow. Patients who are candidates for percutaneous procedures must have occlusions or stenoses that are accessible to the catheter. Reocclusion may occur, and the procedure may be repeated. Some patients are occlusion free for up to 3 to 5 years, whereas others may experience reocclusion within a year. During percutaneous vascular intervention, intravascular stents (wire meshlike devices) are usually inserted to ensure adequate blood flow in a stenosed vessel. Candidates for stents are patients with stenosis of the common or external iliac arteries. Stents are also available to effectively treat superficial femoral artery (SFA) disease. Patients have these procedures in sameday surgery or ambulatory care centers. Another arterial technique to improve blood flow to ischemic legs in people with PAD is mechanical rotational abrasive atherectomy. The Rotablator device is designed to scrape plaque from inside the artery while minimizing damage to the vessel surface and is useful at the popliteal artery and below. Most patients receive anticoagulant or antiplatelet therapy such as heparin or clopidogrel, before and/or during the procedure. An antiplatelet drug may also be prescribed after the procedure to prevent arterial clotting. The most common time frame for the administration of the antiplatelet is 1 to 3 months following the procedure. However, this time frame is highly variable Arterial revascularization Arterial revascularization is the surgical procedure most commonly used to increase arterial blood flow in an affected limb. Surgical procedures are classified as inflow or outflow. Inflow procedures involve bypassing arterial occlusions above the superficial femoral arteries (SFAs). Outflow procedures involve surgical bypassing of arterial occlusions at or below the SFAs. For those who have both inflow and outflow problems, the inflow procedure (for larger arteries) is done before the outflow repair. Inflow procedures include aortoiliac, aortofemoral, and axillofemoral bypasses. Outflow procedures include femoropopliteal and femorotibial bypasses. Inflow procedures are more successful, with less chance of reocclusion or postoperative ischemia. Outflow procedures are less successful in relieving ischemic pain and are associated with a higher incidence of reocclusion. Graft materials for bypasses are selected on an individual basis. For outflow procedures, the preferred graft material is the patient’s own (autogenous) saphenous vein. However, some patients experience coronary artery disease and may need this vein for coronary artery bypass. When the saphenous vein is not usable, the cephalic or basilic arm veins may be used. Grafts made of synthetic materials have also been used when autogenous veins were not available. Drug Management of PAD Medications Pentoxifylline (Trental) – for intermittent claudication ↑ Erythrocyte flexibility ↓ Blood viscosity Cilostazol (Pletal) – vasodilator with some antiplatelet activity ASA or other antiplatelet (Plavix) ACE inhibitors Ramipril (Altace) ↓ Cardiovascular morbidity ↓ Mortality ↑ Peripheral blood flow ↑ ABI ↑ Walking distance Characteristics of Arterial Ulcers Located in areas of pressure, tips of toes Very painful Deep, may involve joint Usually circular in appearancedvt Wound base pale to black Little, if any, edema Surgical Management Patients with severe rest pain or claudication that interferes with the ability to work or threatens loss of a limb become surgical candidates. Arterial revascularization is the surgical procedure most commonly used to increase arterial blood flow in an affected limb. Surgical procedures are classified as inflow or outflow. Inflow procedures involve bypassing arterial occlusions above the superficial femoral arteries (SFAs). Outflow procedures involve surgical bypassing of arterial occlusions at or below the SFAs. For those who have both inflow and outflow problems, the inflow procedure (for larger arteries) is done before the outflow repair. Inflow procedures include aortoiliac, aortofemoral, and axillofemoral bypasses. Outflow procedures include femoropopliteal and femorotibial bypasses. Inflow procedures are more successful, with less chance of reocclusion or postoperative ischemia. Outflow procedures are less successful in relieving ischemic pain and are associated with a higher incidence of reocclusion. Graft materials for bypasses are selected on an individual basis. For outflow procedures, the preferred graft material is the patient’s own (autogenous) saphenous vein. However, some patients experience coronary artery disease and may need this vein for coronary artery bypass. When the saphenous vein is not usable, the cephalic or basilic arm veins may be used. Grafts made of synthetic materials have also been used when autogenous veins were not available. Preoperative Deep breathing every 1-2 hr Intraoperative Monitor for graft occlusion (emergency) Postoperative Treatment of graft occlusion Monitor for compartment syndrome Assess for infection Preoperative Care Preparing the patient for surgery is similar to procedures described for general or epidural anesthesia (see Chapter 9). Documentation of vital signs and peripheral pulses provides a baseline of information for comparison during the postoperative phase. Depending on the surgical procedure, the patient may have one or more IV lines, urinary catheter, central venous catheter, and/or arterial line. To prevent postoperative infection, antibiotic therapy is typically given before the procedure. Operative Procedures The anesthesia provider places the patient under general, epidural, or spinal anesthesia. Epidural or spinal induction is preferred for older adults to decrease the risk for cardiopulmonary complications in this age-group. If arterial bypass is to be accomplished by autogenous grafts, the surgeon removes the veins through an incision. The blocked artery is then exposed through an incision, and the replacement vein or synthetic graft material is sutured above and below the occlusion to increase blood flow around the occlusion. For conventional open aortoiliac and aortofemoral bypass (AFB) surgery, the surgeon makes a midline incision into the abdominal cavity to expose the abdominal aorta, with additional incisions in each groin (Fig. 33.5). Graft material is tunneled from the aorta to the groin incisions, where it is sutured in place. In an open axillofemoral bypass (Fig. 33.6), the surgeon makes an incision beneath the clavicle and tunnels graft material subcutaneously with a catheter from the chest to the iliac crest, into a groin incision, where it is sutured in place. Neither the thoracic nor the abdominal cavity is entered. For that reason, the axillofemoral bypass is used for high-risk patients who cannot tolerate a procedure requiring abdominal surgery. Minimally invasive surgical techniques are beginning to be performed by vascular surgeons using robotic-assisted laparoscopic procedures. These newer surgical techniques require extensive training and further research data to determine their usefulness. Aortoiliac and Aortofemoral Bypass The image to the right shows how in aortoiliac and aortofemoral bypass surgery, a midline incision into the abdominal cavity is required, with an additional incision in each groin. Patients who have undergone conventional aortoiliac or aortofemoral bypass are NPO status for at least 1 day after surgery to prevent nausea and vomiting, which could increase intra-abdominal pressure. Those who have undergone bypass surgery of the lower extremities not involving the aorta or abdominal wall (femoropopliteal or femorotibial bypass) may remain NPO until the first postoperative day, when they are allowed clear liquids. Warmth, redness, and edema of the affected extremity are often expected outcomes of surgery as a result of increased arterial perfusion Post operative: Deep breathing every 1 to 2 hour Monitor for graft occlusion (emergency) Treatment of graft occlusion Monitor for compartment syndrome Assess for infection Postoperative Care Thorough and ongoing nursing assessment for postoperative arterial revascularization patients is crucial to detect complications. Deep breathing every 1 to 2 hours and using an incentive spirometer are essential to prevent respiratory complications Axillofemoral Bypass To promote graft patency, monitor the patient’s blood pressure and notify the surgeon if the pressure increases or decreases beyond the patient’s baseline. Hypotension may indicate hypovolemia, which can increase the risk for clotting. Range of motion of the operative leg is usually limited, with no bending of the hip and knee. Consult with the surgeon on a case-by-case basis regarding limitations of movement, including turning. Patients having open procedures may be restricted to bedrest for 24 hours or longer after surgery to prevent disruption of the suture lines. Patients having minimally invasive surgery (MIS) may be ambulatory and eat within the day of surgery. Pain and surgical complications tend to occur less often in patients who have MIS procedures. Emergency thrombectomy (removal of the clot), which the surgeon may perform at the bedside, is the most common treatment for acute graft occlusion. Thrombectomy is associated with excellent results in prosthetic grafts. Results of thrombectomy in autogenous vein grafts are not as successful and often necessitate graft revision and even replacement. Local intra-arterial thrombolytic (clot-dissolving) therapy with an agent such as tissue plasminogen activator (t-PA) or an infusion of a platelet inhibitor such as abciximab may be used for acute graft occlusions. This therapy is provided in select settings in which health care providers are experts in its use. Other antiplatelet drugs such as the glycoprotein IIb/IIIa inhibitors tirofiban and eptifibatide may be used as alternatives. The health care provider considers these therapies when the surgical alternative (e.g., thrombectomy with or without graft revision or replacement) carries high morbidity or mortality rates or when surgery for this type of occlusion has traditionally yielded poor results. Closely assess the patient for manifestations of bleeding if thrombolytics are used. Graft or wound infections can be life threatening. Use sterile technique when providing incisional care and observe for symptoms of infection. Assess the area for induration, erythema, tenderness, warmth, edema, or drainage. Also monitor for fever and leukocytosis (increased serum white blood cell count). Notify the surgeon promptly if any of these symptoms occur. Patients having conventional open bypass procedures are usually hospitalized for 5 to 7 days. Those having MIS procedures usually have shorter stays of 2 or 3 days Peripheral arterial disease (PAD) is a chronic, long-term problem with frequent complications. Patients may benefit from a case manager who can follow them across the continuum of care. The desired outcome is that the patient can be maintained in the home. Management at home often requires an interprofessional team approach, including several home care visits. See the Home Care Considerations: The Patient With Peripheral Vascular Disease box for home care of patients with peripheral vascular disease. Instruct patients on methods to promote vasodilation. Teach them to avoid raising their legs above the level of the heart unless venous stasis is also present. Provide written and oral instructions on foot care and methods to prevent injury and ulcer development. See the Patient and Family Education: Preparing for SelfManagement: Foot Care for the Patient With Peripheral Vascular Disease box. Patients who have had surgery require additional instruction on incision care (see Chapter 9). Encourage all patients to avoid smoking, limit dietary fat intake, and increase protein intake (Jellinger et al., 2017). Remind them to drink adequate fluids to prevent dehydration. Patients with chronic arterial obstruction may fear recurrent occlusion or further narrowing of the artery. They often fear that they might lose a limb or become debilitated in other ways. Indeed, chronic PAD may worsen, especially in those with diabetes mellitus. Reassure them that participation in prescribed exercise, nutrition therapy, and drug therapy, along with cessation of smoking, can limit further formation of atherosclerotic plaques. Patients with arterial compromise or surgery may need assistance from the family, another caregiver, or a home care aide with ADLs if activity is limited by pain. They may need to limit or avoid stair climbing, depending on the severity of disease. Those who have undergone surgery may require a home care nurse to help with incision care. In collaboration with the case manager, arrange for home care resources before discharge. Acute Peripheral Arterial Occlusion Pathophysiology overview: embolus May have severe pain below the level of occlusion Although chronic peripheral arterial disease (PAD) progresses slowly, the onset of acute arterial occlusion is sudden and dramatic. An embolus (piece of a clot that travels and lodges in a new area) is the most common cause of peripheral occlusions, although a local thrombus may be the cause. Occlusion may affect the upper extremities, but it is more common in the lower extremities. Emboli originating from the heart are the most common cause of acute arterial occlusions. Most patients with an embolic occlusion have had an acute myocardial infarction (MI) and/or atrial fibrillation within the previous weeks. Six Ps of Arterial Insufficiency Pain Poikilothermia (coolness) Paralysis Pallor Paresthesia Pulselessness Patients with an acute arterial occlusion describe severe pain below the level of the occlusion that occurs even at rest. The affected extremity is cool or cold, pulseless, and mottled. Small areas on the toes may be blackened or gangrenous due to lack of perfusion. The primary health care provider must initiate treatment promptly to avoid permanent damage or loss of an extremity. Anticoagulant therapy with unfractionated heparin (UFH) is usually the first intervention to prevent further clot formation. The patient may undergo angiography. A surgical thrombectomy or embolectomy with local anesthesia may be performed to remove the occlusion. The health care provider makes a small incision, which is followed by an arteriotomy (a surgical opening into an artery). A catheter is inserted into the artery to retrieve the embolus. It may be necessary to close the artery with a synthetic or autologous (patient’s own blood vessel) patch graft. Acute Peripheral Arterial Occlusion: Interventions: Take Action The primary health care provider must initiate treatment promptly to avoid permanent damage or loss of an extremity. Anticoagulant therapy with unfractionated heparin (UFH) is usually the first intervention to prevent further clot formation. The patient may undergo angiography. A surgical thrombectomy or embolectomy with local anesthesia may be performed to remove the occlusion. The health care provider makes a small incision, which is followed by an arteriotomy (a surgical opening into an artery). A catheter is inserted into the artery to retrieve the embolus. It may be necessary to close the artery with a synthetic or autologous (patient’s own blood vessel) patch graft. Alterations in comfort should significantly diminish after the surgical procedure, although mild incisional pain remains. Watch closely for complications caused by reperfusing the artery after thrombectomy or embolectomy, either of which includes spasms and swelling of the skeletal muscles. Swelling of the skeletal muscles can result in compartment syndrome. Compartment syndrome occurs when tissue pressure within a confined body space becomes elevated and restricts blood flow. The resulting ischemia can lead to tissue damage and eventually tissue death. Assess the motor and sensory function of the affected extremity. Monitor for increasing pain, swelling, and tenseness. Report any of these symptoms to the health care provider immediately. Fasciotomy (surgical opening into the tissues) may be necessary to prevent further injury and save the limb. The use of systemic thrombolytic therapy for acute arterial occlusions has been disappointing because bleeding complications often outweigh the benefits obtained. Catheter-directed intra-arterial thrombolytic therapy with fibrinolytics, such as alteplase or t-PA, has emerged as an alternative to surgical treatment in selected settings. A catheter is placed percutaneously (through the skin) into the artery with or without ultrasound guidance by the vascular surgeon or interventional radiologist. The tip of the catheter is embedded in the clot to directly deliver the thrombolytic infusion until the clot dissolves, which can take 24 to 36 hours. During infusion, monitor the patient for complications such as bleeding and hemorrhagic stroke. Maintaining a normal blood pressure is essential in preventing a potential stroke. As the clot dissolves, the patient typically experiences severe pain due to reperfusion that requires patient-controlled analgesia (PCA) Aneurysms of Central Arteries Pathophysiology overview Aneurysm—permanent localized dilation of artery, enlarging artery to twice its normal diameter An aneurysm is a permanent localized dilation of an artery, which enlarges the artery to at least two times its normal diameter. It may be described as fusiform (a diffuse dilation affecting the entire circumference of the artery) or saccular (an outpouching affecting only a distinct portion of the artery). Aneurysms may also be described as true or false. In true aneurysms, the arterial wall is weakened by congenital or acquired problems. False aneurysms occur as a result of vessel injury or trauma to all three layers of the arterial wall. Dissecting aneurysms differ from aneurysms in that they are formed when blood accumulates in the wall of an artery. Aneurysms tend to occur at specific anatomic sites (Fig. 33.7), most commonly in the abdominal aorta. They often occur at a point where the artery is not supported by skeletal muscles or on the lines of curves or flexion in the arterial tree. This chapter discusses aneurysms of the central arteries. Brain aneurysms are discussed in Chapter 41. An aneurysm forms when the middle layer (media) of the artery is weakened, producing a stretching effect in the inner layer (intima) and outer layers of the artery. As the artery widens, tension in the wall increases; and further widening occurs, thus enlarging the aneurysm and increasing the risk for arterial rupture. Elevated blood pressure can also increase the rate of aneurysmal enlargement and risk for early rupture. When dissecting aneurysms occur, the aneurysm enlarges, blood is lost, and blood flow to organs is diminished. Aneurysms can cause symptoms by exerting pressure on surrounding structures or by rupturing. Rupture is the most frequent complication and is life threatening because abrupt and massive hemorrhagic shock results. Thrombi within the wall of an aneurysm can also be the source of emboli in distal arteries below the aneurysm. Atherosclerosis is the most common cause of aneurysms, with hypertension, hyperlipidemia, and cigarette smoking being contributing factors. Age, gender, and family history also play a role Description Fusiform Saccular True False Dissecting Arterial Aneurysms *common anatomic sites of arterial aneurysms. Aneurysms of Central Arteries: Assessment: Recognize Cues Sometimes asymptomatic AAA Gnawing pain with abdominal, flank, or back pain Pulsation in upper abdomen Detectable = at least 5 cm in diameter Rupture symptoms = severe sudden pain in back, low abdomen; radiates to groin, buttocks, legs Critical illness – at risk for hypovolemic shock caused by hemorrhage Abdominal aortic aneurysms (AAAs) account for most aneurysms, are commonly asymptomatic, and frequently rupture. Most of these are located between the renal arteries and the aortic bifurcation (dividing area) Most patients with abdominal or thoracic aneurysms are asymptomatic when their aneurysms are first discovered by routine examination or during an imaging study performed for another reason. However, a few patients do have symptoms that bring them to their primary health care provider or the emergency department. Assess patients with a known or suspected abdominal aortic aneurysm (AAA) for abdominal, flank, or back pain. Pain is usually described as steady with a gnawing quality, unaffected by movement, and lasting for hours or days. A pulsation in the upper abdomen slightly to the left of the midline between the xiphoid process and the umbilicus may be present. A detectable aneurysm is at least 5 cm in diameter. Auscultate for a bruit over the mass, but avoid palpating the mass because it may be tender, and there is risk for rupture! If expansion and impending rupture of an AAA are suspected, assess for severe pain of sudden onset in the back or lower abdomen, which may radiate to the groin, buttocks or legs. Patients with a rupturing AAA are critically ill and are at risk for hypovolemic shock caused by hemorrhage. Signs and symptoms include hypotension, diaphoresis, decreased level of consciousness, oliguria (scant urine output), loss of pulses distal to the rupture, and dysrhythmias. Retroperitoneal hemorrhage manifests with hematomas in the flanks (lower back). Rupture into the abdominal cavity causes abdominal distention. TAA Back pain Shortness of breath Difficulty swallowing Not often detected by physical assessment May have a mass above the suprasternal notch Thoracic aortic aneurysms (TAAs) are not quite as common and are frequently misdiagnosed. They are typically discovered when advanced imaging is used to assess other conditions. TAAs commonly develop between the origin of the left subclavian artery and the diaphragm. They are located in the descending, ascending, and transverse sections of the aorta. They can also occur in the aortic arch and are very difficult to manage surgically. When a thoracic aortic aneurysm (TAA) is suspected, assess for back pain and manifestations of compression of the aneurysm on adjacent structures. Signs include shortness of breath, hoarseness, and difficulty swallowing. TAAs are not often detected by physical assessment, but occasionally a mass may be visible above the suprasternal notch. Assess the patient with suspected rupture of a thoracic aneurysm for sudden and excruciating back or chest pain. Hypovolemic shock also occurs with TAA. Computed tomography (CT) scanning with contrast is the standard tool for assessing the size and location of an abdominal or thoracic aneurysm. Ultrasonography is also used. Diagnostic Assessment Nonsurgical management X-ray “eggshell” appearance • Monitor aneurysm growth • Maintain BP at normal level to decrease risk of rupture Ultrasonography CT Aortic arteriography The desired outcome of nonsurgical management is to monitor the growth of the aneurysm and maintain the blood pressure at a normal level to decrease the risk for rupture. Patients with hypertension are treated with antihypertensive drugs to decrease the rate of enlargement and the risk for early rupture. For those with small or asymptomatic aneurysms, frequent ultrasound or CT scans are necessary to monitor the growth of the aneurysm. Emphasize the importance of following through with scheduled tests to monitor the growth. Also explain the signs and symptoms of aneurysms that need to be promptly reported. Surgical Management Abdominal aortic aneurysm resection Endovascular repair Thoracic aortic aneurysm repair Surgical management of an aneurysm may be an elective or an emergency procedure. For patients with a rupturing abdominal aortic or a thoracic aneurysm, emergency surgery is performed. Patients with smaller aneurysms that are producing symptoms are advised to have elective surgery. Those with smaller aneurysms that are not causing symptoms are treated nonsurgically until symptoms occur or the aneurysm enlarges. The most common surgical procedure for AAA has traditionally been a resection or repair (aneurysmectomy). However, the mortality rate for elective resection is high and markedly increases for emergency surgery. Endovascular stent grafts have improved mortality rates and shortened the hospital stay for select patients who need AAA repair. The repair of AAAs with endovascular stent grafts is the procedure of choice for almost all patients on an elective or emergent basis. Stents (wirelike devices) are inserted percutaneously (through the skin), avoiding abdominal incisions and therefore decreasing the risk for a prolonged postoperative recovery. Postoperative care is similar to care required after an arteriogram (angiogram). Different designs of endovascular stent grafts are used, depending on the anatomic involvement of the aneurysm. The stent graft is flexible with either Dacron or polytetrafluoroethylene (PTFE) material. It is inserted through a skin incision into the femoral artery by way of a catheter-based system. The catheter is advanced to a level above the aneurysm away from the renal arteries. The graft is released from the catheter, and the stent graft is placed with a series of hooks. This procedure is done in collaboration with the vascular surgeon, interventional radiologist, operating suite team, and at some centers the vascular medicine physician. Aneurysms of Central Arteries: Interventions: Take Action Size and presence of symptoms determines patient management Nonsurgical management Surgical management TAA repair Postoperative Care Assess vital signs at least hourly. Assess the patient hourly for sensation and motion Patient must follow instructions Aneurysms of the Peripheral Arteries Femoral and popliteal aneurysms Do NOT palpate the mass Symptoms Limb ischemia Diminished or absent pulses Cool to cold skin Pain Treatment: Surgery Postoperative care: Report sudden development of pain or extremity discoloration Although femoral and popliteal aneurysms are not common, they may be associated with an aneurysm in another location of the arterial tree (see Fig. 33.7). To detect a popliteal aneurysm, assess for a pulsating mass in the popliteal space. To detect a femoral aneurysm, observe a pulsatile mass over the femoral artery. To prevent its rupture, do not palpate the mass! Evaluate both extremities because more than one femoral or popliteal aneurysm may be present. The patient may have symptoms of limb ischemia (decreased perfusion ), including diminished or absent pulses, cool to cold skin, and pain. Alterations in comfort may be present if an adjacent nerve is compressed. The recommended treatment for either type of aneurysm, regardless of size, is surgery because of the risk for thromboembolic complications. To treat a femoral aneurysm, the surgeon removes the aneurysm and restores circulation using a synthetic or an autogenous saphenous vein graft-stent repair. Most surgeons prefer to bypass rather than resect a popliteal aneurysm. After surgery, monitor for lower-limb ischemia. Palpate pulses below the graft to assess graft patency. Often Doppler ultrasonography is necessary to assess blood flow when pulses are not palpable. Report sudden development of pain or discoloration of the extremity immediately to the surgeon because it may indicate graft occlusion. Aortic Dissection Pathophysiology Overview Formerly called “dissecting aneurysm” Sudden tear in aortic intima; blood enters aortic wall Highly lethal, emergent situation Aortic dissection is thought to be caused by a sudden tear in the aortic intima, allowing blood to enter the aortic wall. Degeneration of the aortic media may be the primary cause for this condition, with hypertension y p y yp being an important contributing factor. It is often associated with genetic connective tissue disorders such as Marfan syndrome. It occurs also in middle-age and older people, peaking in adults in their 50s and 60s. Men are more commonly affected than women. The circulation of any major artery arising from the aorta can be impaired in patients with aortic dissection; therefore this condition is highly lethal and represents an emergency situation. Although the ascending aorta and descending thoracic aorta are the most common sites, dissections can also occur in the abdominal aorta and other arteries. Aortic Dissection : Interventions: Take Action Insert two large-bore IV catheters 0.9% sodium chloride and medication Indwelling catheter Subsequent treatment depends on dissection location The expected outcomes for emergency care for a patient with an aortic dissection are increased comfort and reduction of systolic blood pressure to 100 to 120 mm Hg. Make sure that the patient has two largebore IV catheters to infuse 0.9% sodium chloride and give medication. Insert an indwelling urinary catheter. The health care provider prescribes IV morphine sulfate to relieve pain and an IV beta blocker, such as esmolol, p p to lower heart rate and blood pressure (Black & Manning, 2018). If this regimen is not effective, nitroprusside or nicardipine hydrochloride may be used. Subsequent treatment depends on the location of the dissection. Patients receive continued medical treatment for uncomplicated distal dissections and surgical treatment for proximal dissections. For longterm medical treatment, the recommended target for blood pressure is less than 120/80 mm Hg (Black & Manning, 2018). Beta blockers (e.g., propranolol) and calcium channel antagonists (e.g., amlodipine) are prescribed to assist with blood pressure maintenance once the patient is stabilized. Patients having surgical intervention for a proximal dissection typically require cardiopulmonary bypass (CPB) (see Chapter 35). The surgeon removes the intimal tear and sutures edges of the dissected aorta. Usually a synthetic graft is used. Peripheral Venous Disease Thrombus formation Skeletal muscles that do not contract to help pump blood in the veins Defective valves To function properly, veins must be patent (open) with competent valves. Vein function also requires the assistance of the surrounding muscle beds to help pump blood toward the heart. If one or more veins are not operating properly, they become distended, and signs and symptoms occur. Three health problems alter the blood flow in veins: • Thrombus formation (venous thrombosis) can lead to pulmonary embolism (PE), a life-threatening complication. Venous thromboembolism (VTE) is the current term that includes both deep vein thrombosis (DVT) and PE. • Defective valves lead to venous insufficiency and varicose veins, which are not life threatening but are problematic. • Skeletal muscles do not contract to help pump blood in the veins. This problem can occur when weight bearing is limited or muscle tone decreases. Venous Thromboembolism Pathophysiology Overview Thrombus Phlebothrombosis Thrombophlebitis DVT Venous thromboembolism (VTE) is one of health care’s greatest challenges and includes both thrombus and embolus complications. A thrombus (also called a thrombosis) is a blood clot believed to result from an endothelial injury, venous stasis, or hypercoagulability. The thrombosis may be specifically attributable to one element, or it may involve all three elements. It is often associated with an inflammatory process. When a thrombus develops, immunity is altered, causing inflammation to occur around the clot, thickening of the vein wall, and possible embolization (the formation of an embolus). Pulmonary embolism (PE) is the most common type of embolus and is discussed in detail in Chapter 29. Phlebothrombosis is a thrombus without inflammation. Thrombophlebitis refers to a thrombus that is associated with inflammation. Thrombophlebitis can occur in superficial veins. However, it most frequently occurs in the deep veins of the lower extremities. Deep vein thrombophlebitis, commonly referred to as deep vein thrombosis (DVT), is the most common type of thrombophlebitis. It is more serious than superficial thrombophlebitis because it presents a greater risk for PE. With PE, a dislodged blood clot travels to the pulmonary artery—a medical emergency! DVT develops most often in the legs but can also occur in the upper arms as a result of increased use of central venous devices. Peripheral Venous Disease: Etiology Virchow’s triad Blood flow stasis Endothelial injury Hypercoagulability Thrombus formation has been associated with stasis of blood flow, endothelial injury, and/or hypercoagulability, known as the Virchow triad. The precise cause of these events remains unknown; however, a few predisposing factors have been identified. The highest incidence of clot formation occurs in patients who have undergone hip surgery, total knee replacement, or open prostate surgery. Other conditions that seem to promote thrombus formation are ulcerative colitis, heart failure, cancer, oral contraceptives, and immobility. Complications of immobility occur during prolonged bedrest such as when a patient is confined to bed for an extensive illness. People who sit for long periods (e.g., on an airplane or at a computer) are also at risk. Phlebitis (vein inflammation ) associated with invasive procedures such as IV therapy can also predispose patients to thrombosis. Peripheral Venous Disease: Assessment: Recognize Cues Assess the patient for a history of any type of VTE. In addition, assess him or her for risks that may be associated with the development of VTE such as prolonged periods of sitting or bedrest, recent surgical procedures, or any factors that may affect coagulation. The Padua Prediction Score (PPS) has been suggested as the best available model for the assessment of the risk of VTE in hospitalized medical patients (Germini et al., 2016). The PPS is meant to assess risk for VTE, not to diagnose VTE. During the nursing assessment, one point is given for each of the characteristics, which include: • Active cancer • Previous VTE (excluding superficial vein thrombosis) • Reduced mobility • Known thrombophilic condition • Recent (≤1 month) trauma and/or surgery • Older adult (≥70 years) • Cardiac and/or respiratory failure • Acute MI and/or ischemic stroke • Acute infection and/or rheumatologic disorder • Obesity (body mass index [BMI] ≥30) • Ongoing hormonal treatment A score of 4 or more indicates that a DVT is likely to occur Physical Assessment/Signs and Symptoms People with DVT may have symptoms or may be asymptomatic. The classic signs and symptoms of DVT are calf or groin tenderness and pain and sudden onset of unilateral swelling of the leg. Pain in the calf on dorsiflexion of the foot (positive Homans sign) appears in only a small percentage of patients with DVT, and false-positive findings are common. Therefore checking a Homans sign is not advised because it is an unreliable tool! Examine the area described as painful, comparing this site with the other limb. Gently palpate the site, observing for induration (hardening) along the blood vessel and for warmth and edema. Redness may also be present Peripheral Venous Disease: Assessment: Recognize Cues Other Diagnostic Assessments Venous duplex ultrasonography Doppler flow studies Impedance plethysmography MRI d-dimer If a definitive diagnosis is lacking from physical assessment findings alone, diagnostic tests may be performed. The preferred diagnostic test for DVT is venous duplex ultrasonography, a noninvasive ultrasound that assesses the flow of blood through the veins of the arms and legs. Doppler flow studies may also be useful in the diagnosis, but they are more sensitive in detecting proximal rather than distal DVT. Normal venous circulation creates audible signals, whereas thrombosed veins produce little or no sound. The accuracy of the scanning depends on the technical skill of the health care professional performing the test. If the test is negative but a DVT is still suspected, a venogram may be needed to make an accurate diagnosis. Impedance plethysmography assesses venous outflow and can detect most DVTs that are located above the popliteal vein. It is not helpful in locating clots in the calf and is less sensitive than Doppler studies. Magnetic resonance direct thrombus imaging, another noninvasive test, is useful in finding a DVT in the proximal deep veins and is better than traditional venography in finding DVT in the inferior vena cava or pelvic veins. A D-dimer test is a global marker of coagulation activation and measures fibrin degradation products produced from fibrinolysis (clot breakdown). The test is used for the diagnosis of DVT when the patient has few clinical signs and stratifies patients into a high-risk category for recurrence. Useful as an adjunct to noninvasive testing, a negative D-dimer test can exclude a DVT without an ultrasound. Peripheral Venous Disease: Analysis: Analyze Cues and Prioritize Hypothesestter Analysis: Interpreting Potential for injury due to complications of VTE and anticoagulation therapy Peripheral Venous Disease: Intervention: Generate Solutions & Take Action surgical management nonsurgical management The focus of managing thrombophlebitis is to prevent complications such as pulmonary emboli, further thrombus formation, and an increase in size of the thrombus. Patients with deep vein thrombosis (DVT) may be hospitalized for treatment, although this practice is changing as a result of the use of newer drugs. Nonsurgical Management DVT is usually treated medically with a combination of rest and drug therapy. Prevention of DVT and other types of VTE is crucial for patients at risk. For those at moderateto-high risk, initiate these interventions to prevent VTE: • Patient education • Leg exercises • Early ambulation • Adequate hydration • Graduated compression stockings • Intermittent pneumatic compression, such as sequential compression devices (SCDs) • Venous plexus foot pump • Anticoagulant therapy Supportive therapy for DVT has typically included bedrest and elevation of the extremity. However, research shows that ambulation does not increase the risk for pulmonary embolus (Lip & Hull, 2018). The risk of pulmonary embolism (PE) associated with more aggressive activity is unknown. The accepted approach is a gradual increase in ambulation as tolerated by the patient. Allowing patients to ambulate may decrease their fear and anxiety about dislodging the clot and life-threatening complications. Teach the patient to elevate his or her legs when in the bed and chair. To help prevent chronic venous insufficiency, instruct patients with active and resolving DVT to wear knee- or thigh-high sequential or graduated compression stockings for an extended period. Be sure to select the correct stocking size for the patient according to the sizing chart provided. Some health care providers prescribe intermittent or continuous warm, moist soaks to the affected area to promote circulation and reduce pain. To prevent the thrombus from dislodging and becoming an embolus, do not massage the affected extremity. Monitor all patients for signs and symptoms of PE, which include shortness of breath, chest pain, and acute confusion (in older adults). Emboli may also travel to the brain or heart, but these complications are not as common as PE. Chapter 29 describes PE manifestations in detail. Drug therapy. Anticoagulants are the drugs of choice for actual DVT and for patients at risk for DVT. However, these drugs are known to cause medical complications and even death. The Joint Commission’s National Patient Safety Goals (NPSGs) include elements of performance to reduce the likelihood of patient harm associated with the use of anticoagulant therapy (TJC, 2018). Medical Management of DVT Low Molecular Weight Heparin (LMWH) Enoxaparin (Lovenox) Dosed based on weight Dose different for treatment of DVT vs prevention of DVT Less bleeding issues More cost effective Low-molecular-weight heparin. Subcutaneous LMWHs such as enoxaparin or dalteparin have a consistent action and are preferred for prevention and treatment of DVT. LMWHs bind less to plasma proteins, blood cells, and vessel walls, resulting in a longer half-life and more predictable response. These drugs inhibit thrombin formation because of reduced factor IIa activity and enhanced inhibition of factor Xa and thrombin. Some patients taking LMWH may be safely managed at home with visits from a home care nurse. Candidates for home therapy must have stable DVT or PE, low risk for bleeding, adequate renal function, and normal vital signs. They must be willing to learn self-injection or have a family member, friend, or home care nurse administer the subcutaneous injections. Some health care providers place the patient on a regimen of IV unfractionated heparin (UFH) for several days and then follow up with an LMWH. In this case, the UFH is discontinued at least 30 minutes before the first LMWH injection. Assess all stools for occult blood. The aPTTs are not checked on an ongoing basis because the doses of LMWH are not routinely adjusted. The anti-Xa factor can be assessed to monitor the effect of the LMWH. Therapeutic range of the anti-Xa factor for LMWH therapy is 0.5 to 1.2 IU/mL (Pagana & Pagana, 2018) Un-fractionated Heparin IV infusion given over 5-7d or until Warfarin brings INR up (2-3) Warfarin (Coumadin) started at the same time Usual dose: 80units/kg bolus followed by 18units/kg/hr Requires monitoring of PTT levels The conventional treatment has been IV unfractionated heparin (UFH) followed by oral anticoagulation with warfarin (Coumadin). However, UFH can be problematic because each patient’s response to the drug is unpredictable and hospital admission is usually required for laboratory monitoring and dose adjustments. The use of low–molecular- weight heparin (LMWH) and the development of novel direct oral anticoagulants (DOACs, also referred to as novel oral anticoagulants [NOACs]) has changed the management of both DVT and PE. Regardless of the approach to anticoagulation, all patients should be assessed before and during anticoagulant therapy for bleeding risk. Unfractionated heparin therapy. Some patients with a confirmed diagnosis of an existing blood clot are started on a regimen of IV UFH therapy. The health care provider prescribes UFH to prevent further clotting which often develops in the presence of an existing clot, and to prevent enlargement of the existing clot. Over a long period of time, the body slowly absorbs the existing clot. Before UFH administration, a baseline prothrombin time (PT), activated partial thromboplastin time (APTT or aPTT), international normalized ratio (INR), complete blood count (CBC) with platelet count, urinalysis, stool for occult blood, and creatinine level are required. Notify the primary health care provider if the platelet count is below 100,000 to 120,000/mm3 , depending on agency protocol. UFH is initially given in a bolus IV dose followed by continuous infusion via pump (Hull et al., 2018). The infusion is regulated by a reliable electronic pump that protects against accidental free flow of solution. The health care provider or clinical pharmacist prescribes concentrations of UFH (in 5% dextrose in water) and the number of units or milliliters per hour needed to maintain a therapeutic aPTT. aPTT is measured at least daily, 6 hours after initiation, and 6 hours after any dose change, and the results are reported to the health care provider as soon as they are available to allow adjustment of heparin dosage. Therapeutic levels of aPTT are usually 1.5 to 2.5 times normal control levels. While most patients who receive UFH are monitored using the aPTT value, the heparin anti-factor Xa (anti-Xa) is sometimes used to monitor and adjust therapy (Zehnder, 2019). The therapeutic range of the anti-Xa factor for UFH is 0.3 to 0.7 IU/mL (Pagana & Pagana, 2018). There is no evidence to suggest that one value is better for monitoring than another, and the aPTT and anti-factor Xa may be used together. Refer to specific agency policy for monitoring protocols. UFH can also decrease platelet counts. Mild reductions are common and are resolved with continued heparin therapy. Severe platelet reductions, although rare, result from the development of antiplatelet bodies within 6 to 14 days after the beginning of treatment. Platelets aggregate into “white clots” that can cause thrombosis, usually in the form of an acute arterial occlusion. The health care provider discontinues heparin administration if severe heparin-induced thrombocytopenia (HIT) (platelet count <150000) or “white clot syndrome,” occurs. Low–molecular-weight heparin (LMWH) is used more commonly today because of the complications involved with UFH. Dabigatran is a direct thrombin inhibitor that may be used as an alternative to heparin or for patients who have had HIT. Like heparin, these drugs increase the risk for bleeding. Monitor hemoglobin, hematocrit, aPTT, platelet count, urinalysis, fecal occult blood test, and blood pressure for indications of this complication. An oral anticoagulant such as warfarin (Coumadin) may also be substituted for heparin if necessary. Ensure that protamine sulfate, the antidote for heparin, is available if needed for excessive bleeding. The Best Practice for Patient Safety & Quality Care: The Patient Receiving Anticoagulant Therapy box highlights information important to nursing care and patient education associated with anticoagulant therapy. Medical Management of DVT Warfarin Used for long-term therapy Antidote = vitamin K Dose individualized Monitor PT/INR levels closely Started on day 1 with LMWH or Un-fractionated heparin Multiple drug and food interactions! Warfarin therapy .If the patient is receiving continuous UFH, warfarin (Coumadin), an oral anticoagulant, may be added. This anticoagulant drug overlap is necessary because heparin and warfarin work differently. Warfarin works in the liver to inhibit synthesis of the four vitamin K– dependent clotting factors and takes 3 to 4 days before it can exert therapeutic anticoagulation. The heparin continues to provide therapeutic anticoagulation until this effect is achieved. IV heparin is then discontinued. Patients receiving LMWH are placed on the oral drug after the first dose. According to the National Patient Safety Goals, therapeutic levels of warfarin must be monitored by measuring the international normalized ratio (INR) at frequent intervals. Because prothrombin times are often inconsistent and misleading, the INR was developed. Most laboratories report both results. Most patients receiving warfarin should have an INR between 1.5 and 2.0 to prevent future DVT and to minimize the risk for stroke or hemorrhage (Pagana & Pagana, 2018). For patients with additional cardiovascular problems or pulmonary embolus, the desired INR is higher, up to 3.5 or 4.0. The health care provider specifies the desired INR level to obtain. Be aware of the critical value for INR according to agency policy (usually greater than 5). Notify the health care provider immediately if your patient’s INR is at a critical value. After obtaining the patient’s baseline INR, warfarin therapy should be started with low doses and gradually titrated up according to the INR. Patients usually receive this drug for at least 3 months or longer after an episode of DVT if no precipitating factors were discovered, with recurrence, or if there are continuing risk factors. Peripheral Venous Disease: Care Coordination and Transition Management Home care: Patients recovering from thrombophlebitis or DVT are ambulatory when they are discharged from the hospital. The primary focus of planning for discharge is to educate the patient and family about anticoagulation therapy. Patients who have experienced DVT may fear recurrence of a thrombus. They may also be concerned about treatment with warfarin and the risk for bleeding. Assure them that the prescribed treatment will help resolve this problem and that ongoing assessment of prothrombin times and INR values decreases the risks for bleeding. Self-management education: Teach patients recovering from DVT to stop smoking and avoid the use of oral contraceptives to decrease the risk for recurrence. Alternative forms of birth control may be used. Most patients are discharged on a regimen of warfarin (Coumadin) or low– molecular-weight heparin (LMWH). Patients receiving subcutaneous LMWH injections at home need instruction on self-injection (see the National Patient Safety Goals: Anticoagulants box). Teach the appropriate caregiver and family members or friends, if necessary, to administer the injections. Instruct patients and their families to avoid potentially traumatic situations, such as participation in contact sports. Provide written and oral information about the signs and symptoms of bleeding (see the Best Practice for Patient Safety & Quality Care: The Patient Receiving Anticoagulant Therapy box). Reinforce the need to report any of these manifestations to the primary health care provider immediately. The anticoagulant effect of warfarin may be reversed by omitting one or two doses of the drug or by the administration of vitamin K. In case of injury, teach patients to apply pressure to bleeding wounds and to seek medical assistance immediately. Encourage them to carry an identification card or wear a medical alert bracelet that states that they are taking warfarin or any other anticoagulant. Instruct patients to tell their dentist and other health care providers that they are taking warfarin before receiving treatment or prescriptions. Prothrombin times are affected by many prescription and over-the-counter drugs such as NSAIDs. Teach patients to avoid high-fat and vitamin K– rich foods (see the Patient and Family Education: Preparing for Self- Management: Food and Drugs That Interfere With Warfarin [Coumadin] box). Remind them to drink adequate fluids to stay well hydrated, avoid alcohol (which can cause dehydration), and avoid sitting for long periods Evaluation: reflecting Evaluate the care of the patient with VTE on the basis of the identified priority problem. The expected outcome is that he or she: • Remains free of injury associated with VTE complications such as pulmonary embolism and bleeding associated with anticoagulation therapy. Venous Insufficiency Result of prolonged venous hypertension that stretches veins and damages valves Leg edema, stasis dermatitis, stasis ulcers Nonsurgical management unless complicated by stasis ulcer Surgical management Venous insufficiency occurs as a result of prolonged venous hypertension that stretches the veins and damages the valves. Valvular damage can lead to a backup of blood and further venous hypertension, resulting in edema and decreased tissue perfusion. With time, this stasis (stoppage) results in venous stasis ulcers, swelling, and cellulitis. The veins cannot function properly when thrombosis occurs or when valves are not working correctly. Venous hypertension can occur in people who stand or sit in one position for long periods (e.g., teachers, office personnel). Obesity can also cause chronically distended veins, which lead to damaged valves. Thrombus formation can contribute to valve destruction. Chronic venous insufficiency also often occurs in patients who have had thrombophlebitis. In severe cases, venous ulcers develop. Venous leg ulcers are a major cause of pain, death, and health care costs. Most venous ulcer care is delivered in the community setting by home care nurses or through selfmanagement Venous ulcers Characteristics of Venous Ulcers Usually located on medial malleolus Tend to be superficial, infrequently painful Irregular borders Wet wounds, wound bed usually granular or yellow fibrous Venous stasis ulcers are slightly more manageable than ulcers resulting from arterial disease. They are chronic in nature, with some patients having the same ulcer for years. Ulcers often heal, only to recur in the same area several years later. Two types of occlusive dressings are used for venous stasis ulcers: oxygen-permeable dressings and oxygen-impermeable dressings. Because the role of atmospheric oxygen in wound healing is controversial, opinions vary with regard to which type of dressing is preferred. An oxygenpermeable polyethylene film and an oxygen-impermeable hydrocolloid dressing (e.g., DuoDERM) are common. Hydrocolloid dressings are left in place for a minimum of 3 to 5 days for best effect. Use medical aseptic technique when changing dressings. If the wound is infected, use Contact Precautions in addition to Standard Precautions. Artificial skin products can be used for difficult-to-heal venous leg ulcers. These first-generation products are very expensive but are laying the foundation in the field, with costs anticipated to come down in the future. Except for cultured epithelial autografts, artificial skins are only temporary. Artificial skin serves as a biologic cover to secrete growth factors to promote more growth factor secretion from the patient’s own skin to speed the wound healing process. If the patient is ambulatory, an Unna boot may be used. An Unna boot dressing is constructed of gauze that has been moistened with zinc oxide. Apply the boot to the affected limb, from the toes to the knee, after the ulcer has been cleaned with normal saline solution. It is then covered with an elastic wrap and hardens like a cast. This promotes venous return and prevents stasis. The Unna boot also forms a sterile environment for the ulcer. The health care provider changes the boot about once a week. Instruct the patient to report increased pain, which indicates that the boot may be too tight. The primary health care provider may prescribe topical agents, such as Accuzyme, to chemically débride the ulcer, eliminating necrotic tissue and promoting healing. Remind patients that they may temporarily feel a burning sensation when the agent is applied. If an infection or cellulitis develops, systemic antibiotics are necessary. Surgery for chronic venous insufficiency is not usually performed because it is not successful. Attempts s at transplanting vein valves have had limited success. Surgical débridement of venous ulcers is similar to that performed for arterial ulcers. The desired outcome for the patient with chronic venous insufficiency is to be managed in the home. For patients with frequent acute complications and repeated hospital admissions, case management can help meet appropriate clinical and cost outcomes. Help patients plan for opportunities and facilities that allow for elevation of the lower extremities in and outside the home. In addition, collaborate with the wound specialist to plan care of the ulcers at home. If the primary health care provider prescribes graduated compression stockings, teach patients to apply these stockings before they get out of bed in the morning and to remove them just before going to bed at night. Also advise them that they will probably need to wear these stockings for the rest of their lives. To improve circulation and aid in weight reduction, collaborate with the physical therapist to prescribe an exercise program on an individual basis. Encourage all patients to maintain an optimal weight and consult with the registered dietitian nutritionist to plan a weight-reduction diet. Patients with venous stasis disease, especially those with venous stasis ulcers, may require long-term emotional support to help them meet longterm needs. They may also need help to cope with necessary lifestyle adjustments, such as possible changes in occupation. Patients with venous stasis ulcers may need the assistance of a home care nurse to perform dressing changes. Those with Unna boots need weekly transportation to their primary health care provider for dressing changes. Collaborate with the case manager to arrange for a sequential compression device (SCD) in the home if the primary health care provider prescribes one. Varicose Veins Distended, protruding veins that appear darkened and tortuous Treatment includes the three Es - Elastic compression hose Exercise Elevation Varicose veins are distended, protruding veins that appear darkened and tortuous. They can occur in anyone, but they are common in adults older than 30 years whose occupations require prolonged standing or heavy physical activity. Varicose veins are also frequently seen in patients with systemic problems (e.g., heart disease), obesity, high estrogen states, and a family history of varicose veins. As the vein wall weakens and dilates, venous pressure increases, and the valves become incompetent (defective), causing venous reflux. The incompetent valves enhance the vessel dilation, and the veins become tortuous and distended. The severity of the disease depends on the extent of the distention and reflux. Telangiectasias (spider veins) are dilated intradermal veins less than 1 to 3 mm in diameter that are visible on the skin surface. Most patients are not bothered by them but may consider them unattractive. Most telangiectasias do not develop into the more severe varicose vein disease. More advanced disease causes venous distention (bulging), edema, a feeling of fullness in the legs, and pruritus (itching). As a result, signs and symptoms of venous insufficiency may occur, including venous stasis ulcers, brown pigmentation from extravasated red blood cells (also called skin staining), and pain. Varicose veins and reflux are diagnosed by simple or duplex ultrasonography. The overall purpose of management for patients with varicose veins is to improve and maintain optimal venous return to the heart and prevent disease progression. Conservative measures are the treatment of choice, including the three Es: elastic compression hose, exercise, and elevation. Graduated compression stockings (GCSs) rely on graduated external pressure to improve venous return by applying pressure to the muscles. They are available in many grades or strengths, ranging from 8 to 50 mm Hg pressure. Exercise increases venous return by helping the muscles pump blood back to the heart. Teach patients to avoid high-impact exercises such as horseback riding and running. Daily walks and ankle flexion exercises while sitting are common exercises that are helpful in promoting circulation. Elevating the extremities as much as possible allows gravity to work with the valves in promoting venous return and preventing reflux. Patients who continue to have pain or unsightly veins despite using the three Es may opt for more invasive approaches. Surgical ligation and/or removal of veins (“stripping”) were the procedures of choice for many years. Sclerotherapy to occlude the affected vessel is also an option. However, newer, less-invasive treatments are more common today. They are less painful and have a shorter recovery time. A common procedure is an endovenous ablation, which occludes the varicose vein, most commonly the saphenous vein. Using ultrasound guidance, the clinician advances a catheter into the vein and injects an anesthetic agent. Then the vessel is ablated (occluded) while the catheter is slowly removed. After the procedure, teach the patient the importance of using a GCS or other form of compression (such as elastic compression bandages) for 24 hours a day, except for showers, for at least the first week. Follow-up ultrasonography ensures that the treated vein is closed. The patient is monitored carefully for the first 6 to 8 weeks to determine how healing has progressed. Some patients require continued use of the three Es for many years, depending on the severity of their disease. Assess the affected limb for vascular status, including any changes in color or temperature of the leg. Monitor for pain, edema, and paresthesias that could indicate complications such as DVT or nerve damage. Nerve damage is usually temporary and minimal; it usually resolves within a few months (Wi