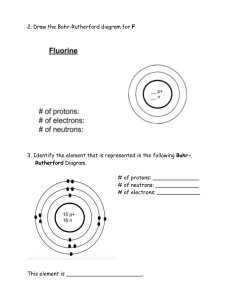
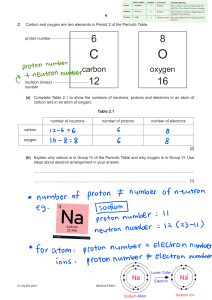
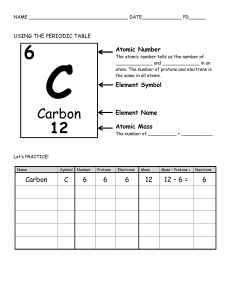
Atomic Structure Practice 1. The 3 particles of the atom are: a.- - - - - - - - - Their charges are: a.- - - - - - - - b. ----------- b. ----------- C. ----------- C.----------- 6. Use a Periodic Table to give the symbol and number of protons in one atom of: Iron ________ Co pper ________ Oxygen _________ Mercur y _________ Krypton --------- Helium --------- - 8. Name the element that has the following numbers of particles: a. 26 electrons, 29 neutrons, 26 protons _______ b. 53 protons, 74 neutrons _______ c. 2 electrons (neutral atom) _______ d. 20 protons __________ e. 86 electrons, 125 neutrons, 82 protons (charged atom) ______ f. 3 protons, 2 electrons, 3 neutrons __________ 10. If you know only the following information can you always determine what the element is? (Yes/No). a. number of protons _____ b. number of neutrons----c. number of electrons in a neutral atom----- d. number of electrons----- 6 11. Determine the number of protons, neutrons and electrons in a neutral atom of Carbon. C Carbon 12.011 14. 12. Argon has three naturally occurring isotopes: argon-36, argon-38, and argon-40. Based on argon’s reported atomic mass, which isotope do you think is the most abundant in nature? Explain 13. Calculate the average atomic mass for neon if its abundance in nature is 90.5% neon-20, 0.3% neon-21, and 9.2% neon 22. Show all work