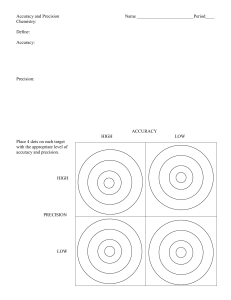
Measurements Review of Basic Concepts Accuracy & Precision Types of Errors Significant Figures & Roundingoff values Precision of measuring devices Measurement of mass & volume Measurements Accuracy – how close a measurement is to the true value Precision – how close a set of measurements are to each other Measurements Measurements Estimating the accuracy and precision of experimental data is extremely important whenever we collect laboratory results because data of unknown quality are worthless. Measure of Accuracy 1. Absolute Error E = xi - xT where xi = experimental value xT = true value or accepted value (literature value) Measure of Accuracy 2. Percent Relative Error % RE = xi - xT xT x 100 For our purpose, % RE < 5% is relatively accurate Measure of Precision 1. Range R = xH - xL where xH = highest experimental value xL = lowest experimental value Measure of Precision 2. Standard Deviation where x = experimental value x = mean n = number of replicates / trials Measure of Precision 2. Standard Deviation • A low standard deviation means that the data is very closely related to the average, thus very reliable (usually less than 5% of the mean) • A high standard deviation means that there is a large variance between the data and the statistical average, thus not as reliable. Exercise Show complete solution, observe correct units and significant figures: ½ crosswise A student determines the boiling point of ethanol in three replicates, obtaining the following results: 78.450, 78.500, 78.390. If the literature value is 78.370, evaluate the accuracy and precision of the data set by calculating the following: a) b) c) d) Absolute error Percent relative error Range Standard deviation Exercise Given: 78.450, 78.500, 78.390 Literature value = 78.370 Mean = (78.450 + 78.500 + 78.390) / 3 = 78.450 a) Absolute Error = 78.450 – 78.370 = 0.080 b) % Relative Error = 0.080 x 100 = 0.10% 78.370 % Relative Error less than 5% of the true value, therefore good accuracy Exercise Given: 78.450, 78.500, 78.390 Literature value = 78.370 Mean = (78.450 + 78.500 + 78.390) / 3 = 78.450 c) Range = 78.500 – 78.390 = 0.110 d) SD = √ (78.450–78.450)2 + (78.500–78.450)2 + (78.390–78.450)2 3-1 SD = 0.060 SD less than 5% of the mean, Therefore good precision Review of Basic Concepts Accuracy & Precision Types of Errors Significant Figures & Roundingoff values Precision of measuring devices Measurement of mass & volume Types of errors Three general types of errors occur in lab measurements: 1. Random error 2. Systematic error, and 3. Gross errors Random or Indeterminate Errors • Random (or indeterminate) errors are caused by uncontrollable fluctuations in variables that affect experimental results • This type of error causes data to be scattered more or less symmetrically around a mean value. • Can be corrected or minimized by doing replicates or multiple trials • Random error in a measurement is reflected by its precision. Systematic or Determinate Errors The errors that affect the accuracy of a result. This type of error causes the mean of a set of data to differ from the accepted value. A systematic error caused the results in a series of replicate measurements to be all high (+ bias) or all low (- bias). Bias has a definite value, an assignable cause and are about the same magnitude for replicate measurements. Bias affects all the data in a set in the same way. How do Systematic Errors Arise? There are three types of systematic errors: 1. Instrumental errors are caused by the imperfections in measuring devices and instabilities in their components. 2. Method errors arise from non-ideal chemical or physical behavior of analytical systems. 3. Personal errors results from the carelessness, inattention, or personal limitations of the experimenter. Instrumental Errors All measuring devices are potential sources of systematic errors. • Calibrated glasswares using glasswares at a temperature that differs significantly from the calibration temperature distortions in container walls due to heating Instrumental Errors • Electronic instruments are also subject to systematic errors errors may emerge as the voltage of a battery-operated power supply decreases with use Errors can also occur if instruments are not calibrated frequently or if they are calibrated incorrectly Method Errors These are errors arising from the following sources • Chemical reagents – ex. wrong choice of chemical indicator in an acid-base titration, non-specific reagent • Chemical reaction – reaction is too slow, incomplete, or side reactions are happening • Experimental procedure – correct reagents, but wrong concentration of reagent specified in the procedure The titration curve of a strong acid with a strong base. Personal Errors Many measurements require personal judgements, judgements of this type are often subject to systematic, unidirectional errors • Estimating the position of a pointer between two scale divisions • Determining the color change at the end point of a titration • Reading the level of a liquid with respect to a graduation in a pipet or buret • Activating a timer Personal Error Parallax Error – error from incorrect reading of volume Effects of Systematic Errors • Constant Errors: Constant Errors does not depend on the size of the quantity measured • Proportional Errors: Proportional errors decrease or increase in proportion to the size of the sample taken for analysis. A common cause of proportional errors is the presence of interfering contaminants in the sample. Detecting Systematic Errors • Systematic instrument errors are usually corrected by calibration. Periodic calibration of equipment is always desirable. • Personal errors can be minimized by care and self-discipline. Errors that result from a known physical disability can usually be avoided by carefully choosing the method. • Method errors or bias of an analytical method is estimated by analyzing standard reference materials. Gross Errors • Gross errors are caused by experimenter carelessness or equipment failure. They occur only occasionally. • Gross error leads to outliers. This error causes one result to differ significantly from the rest of the results (either too large or small) • Often discarded when assessing data Review of Basic Concepts Accuracy & Precision Types of Errors Significant Figures & Rounding-off values Precision of measuring devices Measurement of mass & volume Significant Figures in Measurements - Measures the accuracy and degree of precision of a measuring device - Defined as all the certain digits plus one uncertain digit Significant Figures Two different types of numbers Exact Exact numbers are infinitely important Measured Measured number – measured with a measuring device so these numbers have ERRORS Exact Numbers An exact number is obtained when you count objects or use a defined relationship. • Counting objects are always exact 2 soccer balls 4 pizzas • Exact relationships, predefined values, not measured: 1 foot = 12 inches 1 meter = 100 cm For instance is 1 foot = 12.000000000001 inches? No, 1 foot is EXACTLY 12 inches. Learning Check 1. Exact numbers are obtained by A. using a measuring tool B. counting C. definition 2. Measured numbers are obtained by A. using a measuring tool B. counting C. definition Measurement & Significant Figures Every measurement has a degree of uncertainty. 10’s place is certain 1’s place is certain The 1st decimal place is uncertain What is the Length? 1 2 3 We can’t see the markings between 1.6 - 1.7 We must guess between 1.6 & 1.7 We record 1.67 cm as our measurement The last digit (7) was our guess...stop there 4 cm Learning Check What is stick? 1) 2) 3) the length of the wooden 4.5 cm 4.57 cm 4.574 cm Measured Numbers Do you see why Measured Numbers have error…you have to make that Guess! Significant figures are All certain digits plus one uncertain digit. The last significant figure is only the best possible estimate. Significant figures Length of the wooden stick: 4.57 cm Significant figures Mass measurement: 373.35 g Significant figures 36.5 mL 47 mL 20.38 mL Rules in determining Significant Figures 1. All non-zero digits are significant 1.234 kg 4 significant figures 2. Rules for Zeros depend on position a) Zeros between nonzero digits are significant 606 m 3 significant figures Rules in determining Significant Figures b) Starting zeros are NOT significant 0.008 L 1 significant figure c) Ending zeros i. Significant if there is a decimal point 2.000 mg 4 significant figures ii. Not significant if there is NO decimal point 4002000 g 4 significant figures How many significant figures are in each of the following measurements? 24 mL 2 SF 3001 g 4 SF 0.0320 m3 3 SF 6.4 x 104 molecules 2 SF 560 kg 2 SF Significant Figures Addition or Subtraction The answer must have the same number of decimal places as the original number with the least decimal place 89.332 + 1.1 = 90.432 = 90.4 3.70 - 2.9133 0.7867 round off to 0.79 Significant Figures Multiplication or Division The answer must have the same significant figures as the original number with the smallest number of significant figures 4.51 x 3.6666 = 16.536366 = 16.5 6.8 ÷ 112.04 = 0.0606926 = 0.061 Rounding Off Numbers RULE 1. If the first digit you remove is 4 or less, drop it and all following digits. RULE 2. If the first digit removed is 5 or greater, round up by adding 1 to the last digit kept. If a calculation has several steps, it is best to round off at the END. Rounding Off Numbers Round off the following numbers into 3 SF: 1.5587 1.56 0.0037381 0.00374 13670 13700 128,522 129,000 1.6683 106 1.67 106 Examples of Rounding Round off to 4 SF: 4965.03 4965 780,582 780,600 1999.5 2.000 x 103 Seatwork Determine the number of SF Round-off to 3 SF: 1. 10470 m 6. 0.03458 m 2. 0.00054600 s 7. 10897 g 3. 540 min 8. 1.0539 s 4. 45.000 g 9. 17.000 g 5. 3.50 103 m 10.1.645 10-3 m Review of Basic Concepts Accuracy & Precision Types of Errors Significant Figures & Roundingoff values Precision of measuring devices Measurement of mass & volume Reading a measurement to the correct precision To what precision should a measurement be read? The key is in the graduation or calibration of the measuring device. If the graduation is by tenth or hundredth or thousandth, then the precision is 10% of the graduation. If the graduation is by 2, 0.2, 0.02, then the precision is 25% of the graduation. Reading Temperature Temperature in °C 28.2 + 0.10C Since Δ is 1, then the reader can separate the graduation in their mind to 10 equal increments and therefore the precision is to the tenth of the increment (+ 0.1). Temperature in °F 82.8 + 0.50F Since Δ is 2, then the reader can separate the graduation in their mind to four equal parts. Thus the precision is 25% of the increment (+ 0.5). Reading Lengths Since the smallest increment is 0.1 cm, the the precision of the ruler is + 0.01 cm. 5 3 The length of the rod is 5.50 + 0.01 cm Reading Mass Mass = 126.4 + 0.1 lb Mass = 6.50 + 0.05 g When reading a digital scale, all shown digits are significant. The uncertainty is the last digit on the display. The bathroom scale above changes in increments of 0.1 as the mass is increased. Therefore, the precision of this scale is + 0.1 lb. The digital scale on the right, however, changes in increment of 0.05 g. Therefore, the precision of the digital pocket scale is + 0.05 g. Reading Volumes = 1 mL The precision of this graduated cylinder is 0.1 mL. Vol. = 52.5 + 0.1 mL = 0.2 mL The precision of this graduated cylinder is 0.05 mL. Vol. = 6.60 + 0.05 mL Determine the precision of the given measuring tools? + 0.01 cm + 0.05 mL Determine the precision of the given measuring tools? + 0.01 g Determining the density of the Glycerol sample Initial mass (g) Final mass (g) Mass of added Liquid (g) Trial 1 21.50 + 0.01 25.40 + 0.01 3.90 + 0.02 Volume of liquid (ml) 3.00 + 0.01 Density of liquid (g/cc) 1.30 + 0.01 Review of Basic Concepts Accuracy & Precision Types of Errors Significant Figures & Roundingoff values Precision of measuring devices Measurement of mass & volume Mass mass – measure of the quantity of matter SI unit of mass is the kilogram (kg) 1 kg = 1000 g = 1 x 103 g beam balance electronic balance Measurement of Volume Volume – measure of the three dimensional space occupied by matter SI unit of mass is the cubic meter (m3) Other Units : cubic centimeters (cm3), Cubic decimeters (dm3), liters(l) and milliliters(ml) 1 m3 = 1000 dm3 1 dm3 = 1000 cm3 = 1 L 1 mL = 1 cm3 Measurement of Volume The apparatus chosen depends on the volume and accuracy needed. Measurement of Volume Beaker and Erlenmeyer Flask - Approximate volume measurement Measurement of Volume Graduated Cylinder - More accurate volume measurement Measurement of Volume Burette - Used to accurately measure a volume of reactant needed to react with another reactant Measurement of Volume Pipette - Used to accurately transfer a fixed volume of liquid