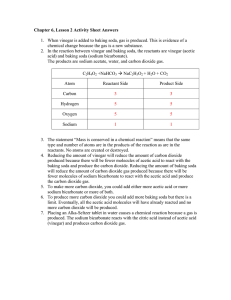
Title Lorem Ipsum Sit Dolor Amet INSTRUCTIONS- Use the vocabulary words to answer the following WORD BANK: Chemical Reactions Exothermic Endothermic Reactions Reactants Products Procedure The reaction of sodium bicarbonate (baking soda) and acetic acid (vinegar) produces carbon dioxide gas, water and sodium acetate (soluble in water). The carbon dioxide gas can originally be seen as bubbles in the solution, but will quickly be released from the solution. The amount of carbon dioxide gas will exceed the space in the bottle, and will move into the deflated balloon, and will inflate it. BEFORE You can sketch or write what are the reactants on this mini lab The reactants in this mini lab are the baking soda and vinegar mixed and created carbon dioxide This happens because the carbon goes to the side that is deflated and fills it up because the side that is inflated can have more air if not it will explode. AFTER You can sketch or write what are the products on this mini lab • The products are vinegar poured in a water bottle and a balloon filled with baking soda You can sketch or write what are the products on this mini lab Is it exothermic or endothermic? It is exo because it releases carbon dioxide and the balloon inflates and the carbon instead of going to the inflated side the mixture is it goes to the deflated side and fills it up. _____________________________________ What sign did you see to say that a chemical reaction occurred. Hint: Think what are the signs to say that a chemical reaction occurs? well the two chemicals where mixed and that made carbon dioxide and made the balloon rise and inflate