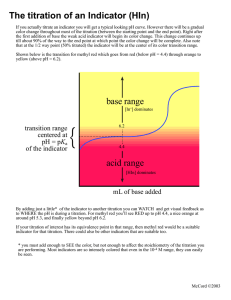
Acid Base Titration Indicators and PH meters . Acid –Base Indicators: Are weak acids and weak bases that change color in acidic and basic media. Examples. Indicator Color in acidic medium Color in basic Used in titration of medium Methyl red Pink Yellow Bromothymol blue Yellow Methyl Orange Red Yellow Strong acid with weak Base Bromophenol Blue Yellow Pink Strong acid with weak base Phenolphthalein Colorless Pink Weak acid with strong base Phenol red Yellow Pink Weak acid with strong base Blue Strong acid with strong base Strong acid with strong base . Measuring PH of a solution. PH can be measured by two methods in the laboratory. 1. Using the universal indicator paper: It is a mixture of indicators that cover the PH from 0 to 14. 2. Using PH-meter: it is an electronic device that measures the PH of a solution by measuring the in the solution. . Each indicator has a PH range called Transition Interval and is defined as the PH range over which the indicator changes color. . Examples of substances and their PH values: Substance PH Substance PH Battery acid 0 Anti acid 8 Stomach acid 2 Baking soda 9 Apple juice 3 Hand soap Black coffee 5 House hold ammonia Pure water 7 Drain cleaner 9-10 11-12 14 . Titration: is the controlled addition and measurement of the amount of a solution of known concentration required to react completely with a measured amount of a solution of unknown concentration. Example . We can use titration to find the concentration of a sample of acid of unknown concentration using a suitable indicator, and vise versa. . Apparatus used in titration. . . The steps of titration. 1. Fill the burette with a solution of NaOH of known concentration (example 0.01 M ) called standard or known solution. 2. Pour a known volume (10 ml) of an acid solution in a conical flask. 3. Add one or two drops of indicator to the acid solution. 4. Add the alkaline solution( NaOH solution) from the burette gradually to the acidic solution in the conical flask and shaken lightly to mix. 5. Keep adding the NaOH solution until the color of indicator changes. 6. Take the reading of the burette example 25 ml ( the volume of NaOH that neutralized the acid ) (.then use the following equation. Ma X Va = Mb X Vb Ma X 10 = 0.01X 25 Ma = 0.01X25 / 10 = 0.025 M is the concentration of the acid. . Example: Calculate the concentration of KOH solution if 15 ml of it needed 17.5 ml of 0.02 M HCl. Suggest an indicator for this titration.