Bond Type Practice: Electronegativity & Lewis Structures
advertisement

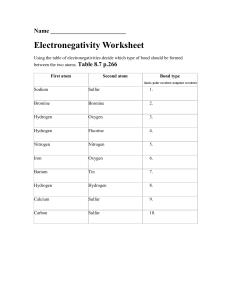
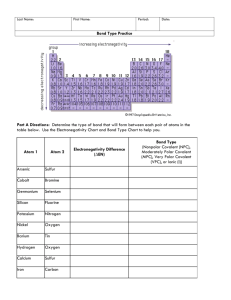
Last Name: First Name: Period: Date: Bond Type Practice The concept of electronegativity was introduced by Linus Pauling in 1932, and this became very useful in explaining the nature of bonds between atoms in molecules. For this work, Pauling was awarded the Nobel Prize in Chemistry in 1954. He also received the Nobel Peace Prize in 1962 for his campaign against above-ground nuclear testing. The greater the electronegativity of an atom of an element, the stronger its attractive pull on electrons. For example, in a molecule of hydrogen bromide ( ), the electronegativity of bromine (2.8) is higher than that of hydrogen (2.1), and so the shared electrons will spend more of their time closer to the bromine atom. Bromine will have a slightly negative charge, and hydrogen will have a slightly positive charge. In a molecule like hydrogen ( ) where the electronegativities of the atoms in the molecule are the same, both atoms have a neutral charge. Part A Directions: Determine the type of bond that will form between each pair of atoms in the table below. Use the Electronegativity Chart and Bond Type Chart to help you. Bond Type Atom 1 Atom 2 Arsenic Sulfur Cobalt Bromine Electronegativity Difference (∆EN) (Nonpolar Covalent (NPC), Moderately Polar Covalent (MPC), Very Polar Covalent (VPC), or Ionic (I)) Germanium Selenium Silicon Fluorine Potassium Nitrogen Nickel Oxygen Barium Tin Hydrogen Oxygen Calcium Sulfur Iron Carbon Part B Directions: Draw the Lewis Dot Structure for each compound below. Then, label each bond as either nonpolar covalent (NPC), moderately polar covalent (MPC), very polar covalent (VPC), or ionic (I). 11. H2O 13. CO2 12. NaCl 14. PF3 Part C Directions: Determine what elements would form each of the 4 bond types with the elements given. Element 15. Boron I VPC MPC NPC Fluorine Oxygen Sulfur Phosphorus 16. Calcium 17. Sulfur