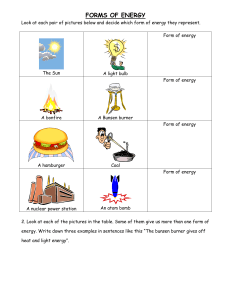
Name:_____________________________________ Date:_________________ Part 1: Lighting a Bunsen Burner Activity PURPOSE: To show the proper technique of lighting and adjusting the Bunsen Burner. Methane is the natural gas used in most labs. Sufficient oxygen is needed for complete combustion. The combustion of methane produces a blue flame producing carbon dioxide and water. When the oxygen supply is insufficient, small carbon particles are produced that form a yellow flame. Bunsen burners were designed to produce a combustible gas-air mixture that produces an efficient, hot flame. A properly adjusted burner flame should have three distinct cones: an outer violet oxidizing flame and an inner blue reducing flame with a cone of unburned gas. The tip of the blue cone is the hottest part of the flame reaching 1500 0C. PROCEDURE: Safety Precautions: This lab requires you to use caution - you need to tie back long hair and roll up long sleeves. Do not touch the barrel of the burner because it gets very hot. Always use tongs when handling hot items in this lab. Also, remember to always turn off the gas when you are finished. Connect the Bunsen Burner tube to the lab bench gas valve 2. Completely close the fuel valve (the dial on the bottom) and the air inlet (the barrel) 3. Open the fuel valve with 1/2 to a full turn 4. When you are ready to light your Bunsen Burner, turn the lab bench gas valve on. 1. **valve is open if the handle is parallel with the outlet Squeeze the striker handles together directly over the burner’s barrel 6. Turn the air inlet (the barrel) to adjust the flame so that it is a small, double blue cone 5. **Do not allow too much air to flow into the barrel since this will just blow out the flame; if this happens, turn off the gas and begin again by closing everything down to the way it was when you started 7. 8. Have your flame approved by the teacher. Teacher Check________ Put the burner out by shutting off the fuel at the lab bench fuel valve; this is the standard procedure for putting out the flame **valve is closed if it is at right angles to the outlet 9. Make sure that everyone in your group has an opportunity to successfully light the burner. Questions: 1. What is needed for burning to occur?______________________ and __________________________ 2. What color flame is the hottest? _____________________________________ 3. Where is the flame the hottest? (Explain using the diagram.) 4. How would you correct a flame that is tall AND bright yellow? 5. Here are safety rules when using a Bunsen burner. Explain why it is important to follow these rules: a. Never turn the gas jet on until you are ready to light the Bunsen burner. b. If your Bunsen burner goes out, turn the gas tap off immediately. c. Tie back your hair and make sure that there are no loose items of clothing. 6. Write the correct letter from the diagram to identify the parts of the Bunsen burner. _____Barrel (where the air and gas are mixed) _____Air intake openings (air enters here) _____Gas flow valve (regulates the flow of gas) _____Gas intake tube (gas enters the burner here) E _____Collar (this adjusts the air intake) _____ Base (supports the burner) Part 2: Scientific Method Lab Purpose: To find out how table salt affects the boiling temperature of water. Write a hypothesis: Materials: 400 mL beaker Ring Stand Wire gauze Bunsen burner Thermometer Stirring Rod Distilled water Table Salt (NaCl) Experimental Procedure: 1. Boil ~250 mL of distilled water using a Bunsen burner. 2. Measure the temperature of the boiling water using a thermometer. Record the highest temperature reading. This is the control. 3. Measure out ~12 g table salt (NaCl) using an electronic balance. 4. Add the measured salt to the boiling water and stir. 5. Measure the temperature of boiling water with salt in it. Record The highest temperature reading. Data and Observations: Post-Lab Questions (answer in complete sentences): 1) What was the independent variable and dependent variable in this experiment? 2) What variables were controlled? 3) Was your hypothesis supported by this experiment? 4) Do you think your results were valid? Explain. 5) What effect(s) will the use of salt in boiling have on cooking?