Classification of Matter Worksheet: Homogeneous vs. Heterogeneous
advertisement

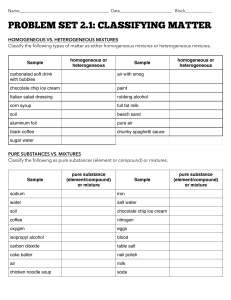
Name: ______________________________ Date: __________________ Period: _______ CLASSIFICATION OF MATTER WORKSHEET HOMOGENEOUS VS. HETEROGENEOUS MATTER Classify the following types of matter as either homogeneous or heterogeneous. 1. carbonated soft drink (w/ bubbles) 2. chocolate chip ice cream 9. air (with smog) 10. paint 3. Italian salad dressing 11. rubbing alcohol 4. corn syrup 12. full fat milk 5. soil 13. beach sand 6. aluminum foil 14. pure air 7. black coffee 15. chunky spaghetti sauce 8. sugar water PURE SUBSTANCES VS. MIXTURES Classify the following as pure substances (element or compound) or mixtures. 1. Sodium (Na) 11. Iron (Fe) 2. Water (H2O) 12. salt water 3. soil 13. chocolate chip ice cream 4. coffee 14. Nitrogen (N) 5. oxygen (O2) 15. Eggs 6. isopropyl alcohol (CH3CHOHCH3) 16. Blood 7. carbon dioxide (CO2) 17. table salt (NaCl) 8. cake batter 18. nail polish 9. air 19. milk 10.chicken noodle soup 20. Soda CLASSIFICATION OF MATTER Check the appropriate categories for the substances listed below. All substances will have a check in more than one column. Substance Heterogeneous Matter Homogeneous Matter Pure Solution Substance Element lead metal (Pb) table salt (NaCl) Kool-Aid drink vegetable soup oxygen gas (O2) distilled water (H2O) Concrete pure gold (Au) brass metal flat 7-Up soda raw egg (cracked open) Air pure iron (Fe) iron rust (Fe2O3) dirt baking soda (NaHCO3) = Compound Mixture