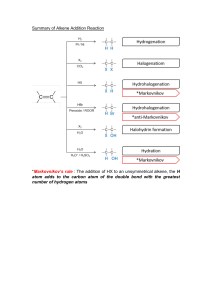
propane C3H8 2-methylpentane C6H14 cycloalkane C6H12 2-methylpropene C4H8 hept-2-ene C7H14 3,3dimethylcyclopentene C7H12 2-bromobutane C4H9Br 2-methylpent-2-ene C6H12 1,2-dibromopropane 2,3-dimethylpentane ethene E/Z isomerism product of addition of HBr to alkene C3H6Br2 product of addition of Br2 to alkene C7H16 9 possible structural isomers C2H4 product of dehydration of ethanol propan-2-ol C3H8O product of the oxidation of pentan-2-ol C5H10O tertiary alcohol 2-methylbutan-2-ol product of the oxidation of butan-1-ol CH3CH2CH2COOH cyclohexanol secondary alcohol ethyl propanoate ethyl methanoate isomer of pentanoic acid formed by methanoic acid and ethanol formed by the addition of HCl to ethene C2H5Cl propanal can be oxidised to propanoic acid ethane C2H6 alkane with a lower boiling point than propane