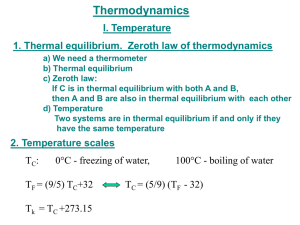
CONCLUSION The following conclusions were obtained after thorough data analysis and interpretation: The students were able to define and understand thermal equilibrium in which the state at which temperature of two bodies in thermal contact becomes equal. Heat, which is a form of energy, is transferred between the objects during the process of reaching thermal equilibrium. The students were able to explain how thermal equilibrium works. When a higher temperature object comes into contact with a lower temperature object, the higher temperature object transfers heat to the lower temperature object. In the absence of loss to other objects, the objects will reach the same temperature and then maintain a stable temperature. In the experiment video, it was observed that the thermometer in the small beaker shows an increase in temperature indicating heat energy. At the same time the thermometer immersed in hot water shows the decrease in temperature as it loses heat energy thus heat energy flows from a body at higher temperature to a body at lower temperature. The students were able to determine if energy could transfer when two objects are in thermal equilibrium with each other. Two objects are in thermal equilibrium if they are in close to one other, allowing one to acquire energy from the other while transferring no net energy. They are in thermal equilibrium even while not in touch if no net energy is transferred between them when they are brought in contact. When two objects are in touch over an extended period of time, they usually come to a state of equilibrium. In other words, in thermal equilibrium, two objects do not exchange energy.