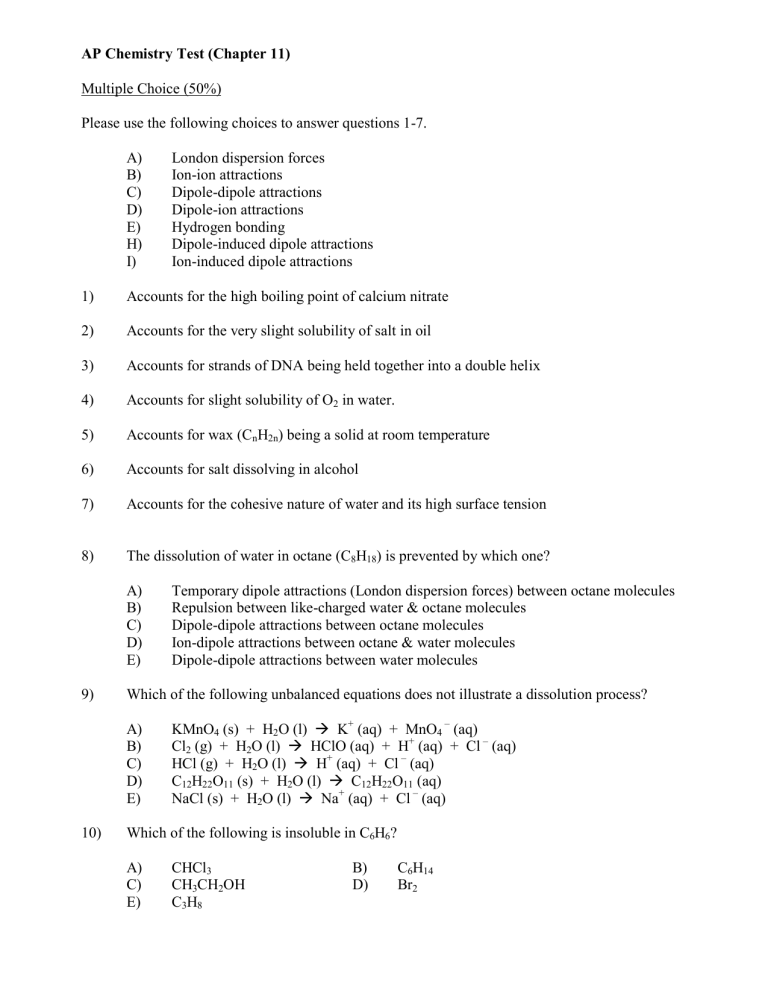
AP Chemistry Test (Chapter 11) Multiple Choice (50%) Please use the following choices to answer questions 1-7. A) B) C) D) E) H) I) London dispersion forces Ion-ion attractions Dipole-dipole attractions Dipole-ion attractions Hydrogen bonding Dipole-induced dipole attractions Ion-induced dipole attractions 1) Accounts for the high boiling point of calcium nitrate 2) Accounts for the very slight solubility of salt in oil 3) Accounts for strands of DNA being held together into a double helix 4) Accounts for slight solubility of O2 in water. 5) Accounts for wax (CnH2n) being a solid at room temperature 6) Accounts for salt dissolving in alcohol 7) Accounts for the cohesive nature of water and its high surface tension 8) The dissolution of water in octane (C8H18) is prevented by which one? A) B) C) D) E) 9) Which of the following unbalanced equations does not illustrate a dissolution process? A) B) C) D) E) 10) Temporary dipole attractions (London dispersion forces) between octane molecules Repulsion between like-charged water & octane molecules Dipole-dipole attractions between octane molecules Ion-dipole attractions between octane & water molecules Dipole-dipole attractions between water molecules KMnO4 (s) + H2O (l) K+ (aq) + MnO4 – (aq) Cl2 (g) + H2O (l) HClO (aq) + H+ (aq) + Cl – (aq) HCl (g) + H2O (l) H+ (aq) + Cl – (aq) C12H22O11 (s) + H2O (l) C12H22O11 (aq) NaCl (s) + H2O (l) Na+ (aq) + Cl – (aq) Which of the following is insoluble in C6H6? A) C) E) CHCl3 CH3CH2OH C3H8 B) D) C6H14 Br2 11) Which of the following is least soluble in water? A) C) 12) Fe2(SO4)3 Na2SO4 0.40 M FeCl2 0.50 M C6H12O6 0.30 M Mg(NO3)2 B) D) 1.00 M NH3 0.20 M KI 78oC and –115oC 82oC and –124oC 78oC and –124oC B) D) 70oC and –110oC 82oC and –110oC Heterogeneous mixture Solution or colloid Solution or compound B) D) Heterogeneous mixture or compound Element or compound Please choose all true statements. A) B) C) D) E) 18) B) D) A clear liquid has no Tyndall effect at 25oC. Which one could be true for the clear liquid? A) C) E) 17) KClO4 Cr(NO3)2 C12H22O11 The normal boiling point (at 1 atm) of pure alcohol is 78oC and the normal freezing point is –115oC. Which one could represent the respective boiling and freezing points when the alcohol contains a solute? A) C) E) 16) Decrease the pressure & decrease the temperature Decrease the pressure & increase the temperature Increase the pressure & decrease the temperature Increase the pressure & increase the temperature Which aqueous solution will have the highest boiling point? A) C) E) 15) CH3CH2OH CH3CH2CH2CH2OH A 0.100 m solution of which one of the following solutes will have the lowest vapor pressure? A) C) E) 14) B) D) Which one will increase the solubility of a gaseous solute in a liquid solvent? A) B) C) D) 13) CH3OH CH3CH2CH2OH All compounds are homogeneous. All solutions are pure. All colloids are heterogeneous. All pure substances are homogeneous. All solutions are homogeneous. Which aqueous solution will have the lowest freezing point? A) C) E) 0.40 M NH3 0.80 M C6H12O6 0.10 M Zn(ClO3)3 B) D) 0.20 M FeBr3 0.60 M NH4NO3 19) Which one is arranged in order of increasing solubility in water (least soluble to most soluble)? A) C) E) 20) CBr4 < CH3OH < KNO3 CH3OH < CBr4 < KNO3 contains as much solvent as it can hold. contains no double bonds. contains dissolved solute in equilibrium with undissolved solute. will rapidly precipitate solute if a seed crystal is added. cannot be attained. Which one best describes the behavior of a seed crystal dropped into an unsaturated solution? A) B) C) D) 22) B) D) A saturated solution _____. A) B) C) D) E) 21) KNO3 < CHCl3 < CBr4 CBr4 < KNO3 < CHCl3 CH3OH < CBr4 < CHCl3 It falls to the bottom of the solution undissolved. It dissolves readily in the solution It rapidly precipitates solute from the solution. It falls to the bottom of the solution & slowly dissolves. What is the concentration of NO3 – in a solution that is 0.900 M Al(NO3)3 ? A) C) E) 0.900 M 1.80 M 2.70 M B) D) 0.300 M 3.60 M Please use the attached solubility graph to answer questions 23-25 23) What mass of NaNO3 will dissolve in 200 g H2O at 10oC to form a saturated solution? A) 24) B) 80 g C) 90 g D) 40 g When a saturated solution of KNO3 in 100 g H2O is cooled from 50oC to 35oC, what mass of KNO3 will crystallize or precipitate from the solution? A) 25) 160 g 58 g B) 85 g C) 143 g D) 27 g Which one is accurately described? A) B) C) D) A supersaturated solution forms when 80 g KNO 3 dissolves in 100 g H2O at 45oC. An unsaturated solution forms when 75 g KNO3 dissolves in 100 g H2O at 45oC. A saturated solution forms when 115 g KNO3 dissolves in 100 g H2O at 60oC. A supersaturated solution forms when 100 g KNO 3 dissolves in 100 g H2O at 60oC. Problems (50%) 1) 200.0 ml of 0.250 M Al(ClO3)3 is mixed with 400.0 ml of 0.400 M Mg(ClO3)2. What is the resulting chlorate ion concentration? 2) A solution is 7.25% H3PO4 by mass. What is the molality of the solution? 3) What mass of antifreeze (ethylene glycol) C2H6O2) must be added to 9.00 L of water in your car’s radiator to produce a solution that will not freeze until -11.3oC. Assume the density of water is exactly 1.00 g/ml. 4) What is the boiling point of a solution that contains 14.5 g Fe(NO3)2 dissolved in 30.0 g water? 5) A chemist is trying to identify a human hormone by its molar mass. A sample weighing 11.976 g was dissolved in 20.0 g benzene. The normal freezing point of benzene is 5.50oC, but the solution containing the hormone had a freezing point of -8.50oC. Kf = 5.12oC ● kg/mol for benzene. What is the molar mass of the hormone?