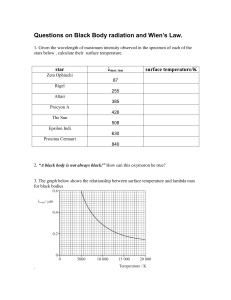
Radiation in a Cavity (Rayleigh, Planck (1900)) Introduction A particularly difficult problem arose during the 19th century – What are the properties of the radiation from a perfect “black body” (an object which absorbs or emits radiation without favouring any particular frequencies)? A good approximation to a black body is a cavity with a small aperture. In the example shown, a hollow sphere with a small hole in it is heated – black body radiation emerges from the aperture. A number of facts were established experimentally: 1. Power emitted per unit area ∝ T4 (Stefan, 1879) Intensity A simple relationship between the wavelength where the peak intensity occurs and the temperature: λmaxT = Constant λ m ax 100 1000 10000 W a v e le n g th (n m ) But – No model to explain these observations! (Classical thermodyanmics can predict Stefan’s law, but not Wien’s displacement law or the form of the spectrum) The Rayleigh-Jeans Law Lord Rayleigh attempted a solution in 1900 (see Atkins, chapter 1 and Appendix 1) Consider an enclosure with perfectly reflecting sides, with dimensions p, q and r. Such a cavity can support modes of radiation provided the wavelength, λ, in each mode satisfies the following relationships: p= lλ mλ nλ where l, m and n are integers. ,q = ,r = 2 2 2 We can then write: ( p 2 + q 2 + r 2 ) = 41 λ2 (l 2 + m 2 + n 2 ) The total number of modes for which the wavelength is greater than or equal to λ is equal to the number of ways l, m and n can be chosen to satisfy the inequalities: 4( p 2 + q 2 + r 2 ) (l + m + n ) ≤ and l , m, n ≥ 0 (negative modes are meaningless!) λ2 2 2 2 Imagine a three-dimensional space with the axes labelled l, m and n. The above inequalities therefore define an ellipsoid in this space, and the number of modes, N, is then simply the volume of the octant of this ellipsoid with l , m, n ≥ 0 . Hence, N = 81 ( Vol. of ellipsoid) = 81 ( 43 πlmn ) pqr λ3 V = 43 π 3 (V = volume of cavity) λ = 43 π The number of modes per unit volume for which the wavelength is greater than or equal to λ is then simply: n( λ ) = 43 π 1 λ3 Differentiating with respect to λ gives the mode density (the number of modes per unit volume per unit wavelength) D( λ )dλ = 4π dλ λ4 Re-writing in terms of frequency, and allowing for the fact that there are two polarisations, we obtain: 8πν 2 D(ν )dν = dν c3 In classical statistical mechanics, every mode carries an average energy kT (k is Boltzmann’s constant and T is the absolute temperature) Hence, the energy density is given by: 8πν 2 U (ν )dν = kTD(ν )dν = kTdν The Rayleigh-Jeans Law. c3 This result agrees well with experiment at low frequencies, but at high frequencies, a problem becomes apparent – The Ultraviolet Catastrophe! The Rayleigh-Jeans Law predicts that the energy density at high frequencies tends to infinity, i.e., all bodies should radiate infinite amounts of energy! This is shown below for a temperature of 6000 K Planck’s Law At about the same time, Max Planck was also working on the problem of black body radiation. He made the assumption that the energy content of each mode is not a continuous variable. Rather, each mode may contain only discrete amounts of energy* (i.e., the energy is quantised) given by E = mhν where m is an integer and h is a universal constant, now known as Planck’s constant. In this case, the average energy of a mode may be calculated from the Boltzmann distribution: ∞ E = ∑ mhνe − ( mhν kT ) m =0 ∞ ∑e − ( mhν kT ) where e −( mhν kT ) is the Boltzmann factor. m =0 ∞ Using the result that ∑x m = (1 − x ) −1 ∞ for x<1, and m=0 ∑ mx m =0 ∞ m = x (∂ ∂x ) ∑ x m we obtain: m =0 hν E = e hν kT −1 Hence, the energy density as a function of frequency is given by: U (ν )dν = 8πhν 3 dν ⋅ hν Planck’s Law 3 c kT e −1 This result gives the correct behaviour at all frequencies, and predicts the properties previously found experimentally. (*It is interesting to note that Planck regarded the oscillators producing the radiation as quantised, rather than the radiation field itself. Later (1931) Planck was to describe his derivation as “an act of desperation” – but this does not refer to the quantisation of energy, rather to his use of Boltzmann statistics which was a controversial subject at the end of the nineteenth century. For more details on Planck’s work on the radiation law, see “The Genesis of Quantum Theory 1899–1913” (Armin Herman), Library ref. 530.12) Some properties of black body radiation 1. Radiant emittance (Power emitted per unit area) c W (ν,T ) = U (ν ) W / m 2 4 2. Power at the peak of the distribution Planck’s Law ⇒ Wien’s Law 2.9 × 10 6 λmax = nm and, emittance at λmax, W max = 129 . × 10 −15 T 5 W / m 2 T 3. Total power output Planck’s Law ⇒ Stefan’s Law Integrating over all frequencies gives: W = 5.68 × 10 −8 T 4 W / m 2 Some examples 1. The Sun (T≈6000K ⇒ λmax ≈ 500nm) 2. Xe flashgun (T ≈ 10000K (transient) ⇒ λmax ≈ 290nm) 3. The universe! (T ≈ 2.7K ⇒ λmax ≈ 1mm) Limitations of thermal sources A hot black body radiator may seem like a good idea for a light source. However, there are a number of problems: 1. Brightness can only be increased by sacrificing monochromaticity or directionality. 2. The radiation cannot be well focused due to poor spatial coherence. 3. Use in interference experiments is limited by poor spatial and temporal coherence. Coherence ⇒ phase relationship between different parts of the wavefront, in both time and space. These quantities are simply related to the bandwidth, ∆ν, of the source. Coherence time, ∆t = 1∆ν . Coherence length, ∆l = c∆t e.g. Source Coherence length Coherence time Tungsten lamp ~ 1 µm ~3 fs Low pressure Hg lamp 3 cm 0.1 ns Cadmium lamp 20 cm 0.7 ns Lasers 0.3 m – 1 km 1 ns – 3 µs