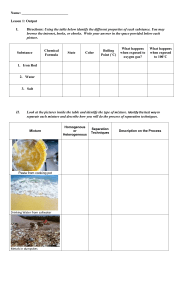
The Classification of Matter Atoms, Elements, Molecules and Compounds PURE SUBSTANCE Something that can not be broken down into simpler matter using physical methods. There are two types of substances… Elements & Compounds. ELEMENTS Are the simplest kind of pure substance. There are ~ 110 elements, each made of a different type of atom (periodic table). Each element has a name and symbol. Not all elements are equally common. Most elements can exist as a single atom (Fe, Cu): But some elements (mostly gases) usually exist as diatomic molecules (groups of 2 atoms). Diatomic Gases Hydrogen H2 Nitrogen N2 Oxygen O2 Fluorine F2 Chlorine Cl2 Bromine Br2 Iodine I2 Allotropes Different structural forms of the same element. Oxygen has 3 allotropes: O Monatomic Oxygen (Single Oxygen Atom) O2 Diatomic Oxygen Molecule O3 Ozone Molecule Allotropes of Carbon Diamond Graphite All are pure carbon. Each has a different structure. Fullerenes Carbon Nanotubes COMPOUNDS Are pure substances that are composed of multiple types of elements (2 or more), chemically bonded to one another. Compounds can not be broken down by physical methods (they can be broken down by chemical reactions). COMPOUNDS Have definite compositions, with element ratios indicated by subscripts (H2O). We call this building block the “water molecule”. COMPOUNDS The properties of compounds are very different from the properties of the elements that make them up! Na + Cl NaCl MIXTURES A mixture is something that CAN be broken down into simpler materials using physical methods. There are 3 possible types of mixtures: 1) Element + Another Element 2) Compound + Another Compound 3) Element + Compound MIXTURES There are 2 main types of mixtures: a) Homogeneous- The parts are distributed evenly. Examples: Salt water; syrup; air; brass. MIXTURES Homogeneous mixtures of liquids are called solutions. Homogeneous mixtures of metallic atoms are called alloys. b) Heterogeneous- The parts are distributed unevenly. Examples: Spaghetti and Meatballs; Water + Oil mixture. We learned that the properties of compounds are TOTALLY DIFFERENT from the properties of the elements that make them up. In contrast, the properties of mixtures ARE related to the properties of the substances that make the mixtures up. Properties of Saltwater Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture Solutions + Alloys no Can it be physically separated? PURE SUBSTANCE no Heterogeneous Mixture yes Can it be chemically decomposed? Compound no Element What are each of these? Atom, Element, Molecule, or Compound Four different molecules: • Which are compounds? Warmup 11/21/11 (R) Types of Particles: Atoms or Molecules or Both? Types of Matter: Elements or Compounds or Mixture? Chemical Symbols Represent Elements Chemical Symbols are either one or two letters. If one letter, it is always capitalized. A chemical symbol implies one atom of that element. If two letters, the first is capitalized, the second is lower case. Chemical Formulas show how many atoms of each element are in one molecule of an element or compound: Chemical Formula # of elements # of atoms # of Carbon total atoms O2 1 2 0 H2O 2 3 0 C3H8O 3 12 3 C6H12O4Cl 4 23 6 Nuts, Bolts, Washers Is it an element, compound, or mixture? What are the element and compound formulas? Nuts, Bolts, Washers Is it an element, compound, or mixture? What are the element and compound formulas? Nuts, Bolts, Washers Is it an element, compound, or mixture? What are the element and compound formulas? WRITING FORMULAS When writing formulas, list the elements in this order: First…bolts. (Bo) Second…nuts. (Nu) Third…washers. (Wa) Example: BoNuWa2 • All pure substances can be represented by a Single Chemical Formula: H2O If it’s really a pure substance, you should only need to write ONE chemical formula to describe it’s composition. How many atoms of each element are present in one molecule of: (NH4)2SO3 N……………. H…………… S……………. O…………… 2 8 1 3 Practice with Vocabulary: Methods of Mixture Separation 1) Mechanical Separation (often by hand) takes advantage of physical properties such as color and shape. Example: Recycling Plastic, Paper, Metal Methods of Mixture Separation 2) Magnetic Separation takes advantage of the physical property of magnetism. Example: Separating Metals in a Scrap Yard Methods of Mixture Separation 3) Filtration takes advantage of the physical property of the state of matter. A screen lets the liquid particles through, but traps the solid particles. Example: Filtering Coffee, Spaghetti A filter can also be used to separate solid particles of different sizes. (ex. a window screen, an air filter, a sand sieve) Methods of Mixture Separation 4) Decanting: To pour off a liquid, leaving another liquid or solid behind. Takes advantage of differences in density. Example: To decant a liquid from a precipitate or water from rice. Methods of Mixture Separation 5 ) Distillation: The separation of a mixture of liquids based on the physical property of boiling point. Example: the distillation of alcohol or oil A distillation tower or “still” Methods of Mixture Separation 6) Evaporation: Vaporizing a liquid and leaving the dissolved solid(s) behind. Used to separate salt solutions. Example: Obtaining sea salt from sea water Methods of Mixture Separation Density Separation: More dense components sink to the bottom and less dense components float. Methods of Mixture Separation 7) Centrifuge: Circular motion helps denser components sink to the bottom faster. Examples: The separation of blood or DNA from blood Methods of Mixture Separation 8) Paper chromatography: Uses the property of molecular attraction to separate a mixture. Different molecules have different attractions for the paper (the stationary phase) vs. the solvent (the mobile phase) Example: the separation of plant pigments and dyes Methods of Mixture Separation Fractional Crystallization: Dissolved substances crystallize out of a solution once their solubility limit is reached as the solution cools. Examples: Growing Rock Candy or the Crystallization of a Magma Chamber