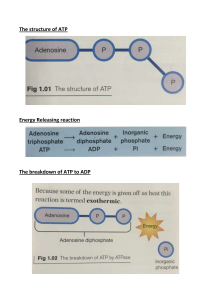
E nergy-rich compou nds 3 3.1 Theoretical part Cell as a complicated astern requires the large amount of free encr for all important activities: the performance of mechanical work in the muscle contraction, and other cellular movemcnts, the active transport of molecules and ions, and the synthesis of macromoleculcs and other biomoleculcs. Free enCf tised iR these processes maintains an org:Inism in a state that is far from the equilibrium, All these processes mentioned above are energetically very demanding and cells use encry u’hich is special for all living systcms — chemical energy. This form of energy is puts in some chemical compounds and is liberated in hydrolysis of some of group which is bounded io this compound by the high energy bonds (- ). There is nothing special about the bonds themselves. There are high-crier bo rids in the sense the i much free ener is released when they are hydrolyscd for the reason gi›cn above. lf âi•h energy bonds are hy0rolysed, prod ecu of reaction go to the energetically lo«’cr siaic hat is expressed as marked change of Gibbs encr ' of system (g . Most of chemical bonds in compounds of orq•anism as ester, pcpiidc and glycosi‹lc bonds arc not high energy and in hydrolqis of these bonds libefaicd energy is about HIS iJ›mo1 of compound. If h's h energy compound (bond) is hy‹lro1;sed much more free energy is liberated (30 —60 kJ,'mol of huh ener compound). Energy-rich molecules are formed by the oxidation of substrates which cull obtains from the environmenc The crealion of enefgy-fiCh compoun‹is in cells is carried on by ihree main ways: t. During oxidation of substrates (for instance glucose) are formed iniermediates ’iih high-energy phosphate group. 2. ATP - the most important high-encry phosphate compound and its phosphoanhydri‹le bonds are referred to as high-energy bonds and is created in the process of oxidative phosphorylation in mitochondria. 5. Some energy-rich compounds are produced so that phosphate is transferred from ATP to anoihef molecule in the reaction which is catalysed by kinase anal high-criers bond is preserved. 3.1.1 Kinds of high-energy bonds Energy-rich compounds in cells compr:se fi e kinds of high-eaer bonds: phosphoanhydride, acvl phosphate, enolphosphaie, guanidine pbospha:e anal ihioesier bonds (Fig. 3.1). Phosphoanhydride bond is formed be: ecn I 'o molecules of pbosphoriC 0Cid (FisPO‹). In hydrolysis of I mol of this bond is liberated approximatelly 30,5 kJ’mol bond. These bonds we can find in nucleotides. Typical representative of high-enery compound with phosphoanhydride bond (diphosphase bond) is ATP (adenosine triphosphaie). In this compound are two high-energy diphosphaie bonds (phosphoanhydride bonds). The ihird phosphate bond bci ecn phosphate and ribose is not enerp’-rich, ii is phosphate ester bond. Similar diphosphate bonds afe in all Si- and iripâosphates of purine and pyrimidinc nucleosi‹lcs. Energy of diphosphate bonds has a ¡;rmi imporNlnce in the metabolism of a cell. ATP sexes as ihe principal immediate donor of free ener in biological systems in most ender*onic reactions of iâc cell, in the active transport of molecules across membranes, muscle contraction, iransmlssion of neo’c impulse, and the oiher processes which requise energy. Despite that fact that ATP is the principal donor of enerp (source of encre’), for some meiabolic paihwajs can be used energy of diphosphate bonds of another nucleosides as GTP (tuanosine triphosyhate) which is donor of energy in the proicosvnthcsis and also in the gluconeogcncsis. L/TP (uridinc iriphosphate) is imporiam nucleoiide in ihe meiabolism of saccharidcs and CTP (q’iid inc triphosphatc) in the mctabolism o( lipiüs. 19 Enolphosphate l›onsd is: formed whcn: phosphate gro,up is a ttached to the hydrox group. «hich is böunüćd to cs rbon with double bond. :CÕHP.õ.UN0 æ,s ș PHQŞPHOAHHYORIOE’!’’ ,AfĘ : ğ ’" ğ 1ïs cncrgy-rich bonü can be transform ATP (this process is: c«I:lüd yhos- :EKBL...: y.tt,PfłbSPHATE t PH ŁI.S^H0E"0L’üY,RU V’ATč’ ö1 ghorvlaiion o/ the substrate). Acy,lph sphate bond iS formed by ICC ÊCa,Cțloțl Of carbo 3’lJc acid n ith phosphate troops. ,In , the hydrolysis is liberated approximately 4 k3im 1 I energy. This type of bond is in Ip-ñis- phosphöglş-ceraië:and is föimëd in the is c*ÎÌ-l'„O GCÄFIO'l8E PHß:ŠPŁIAT”Ë ATP. This tjpe of hi,h-energy bon‹ls is 0 Ć glycolysis ::,and ,can be also transferred from this! compo.und. to ADD° to form PH.0SPH0CREÅ„TI NE xa -»e-o 41, formed also, in the activation of fatty acids and am.ino acids when these react with ATP. Product is acyladenylate resp. aminoacyladenylate. :Gu:aai:d ine ph!osphate bonÒ is .förmed if phösphaie group is attached to guanidine g:roup. Energy of the hydï ,: U‹1 ‹*’ 43 /N *: JP tant cömpouñd with this bond is phosphocieati,ne. êhösphöcrNilfie. is first of all in: muscle celts here is a reserve of energy for this” tïssue. 0g In cells rms type of bond is formed which is one of the.princïyat er›črçctical substr3‹cs oí thc ccll. 3.1.2 Energy-rich compounds in muscle cells The muscle contraction is mechanical work and muscle cell is actua.lly „machine“ where chemical energy of hìghmnergy compounds is changing to mechanical work. As the,principal,söurce ofenefgy,.ìn ihe muscle coRtraciioascwes ATP. In resting mmclethe mmcle cell creates definite level of ATP an4 ratio of ATP:ADP is about 10: 1. Duri•s the muscle work the great amount of ATP is use4: The muscle cell has the eng matic equipment for producing ATP in the glycolysis and also in the oxidative pho,sphorylaiion. la active muscle level of ATP decreases. The reduced enemy charge uf active muscle stimulates the glycogen„breakdown,,glycolysis, citric acid„cycle, and the oxidative phtisphorylaiion. These prticusses are,the relative Contributions to the generation of ATP. In the siäte when the müscle is acii e (vigorously wording) appears the anaerobic condiiioni and so Uhe generation of ATP by the oxidation of substrates is considerably limited. So in the resting state muscle cell uses phosphocreaiine hich contains phosphoguanido group to store high-potential phosphoryl group in the muscle. The concentration of phosphocreatine in the muscle in the resting state is five more times higher than the: level of ATP. R’urliing musclu is able to use ener of phosphoguanido group of phosphocreaiine for the re cncrution uf ATP. Ăhosp1oceatine is /ormad by the reaction: ATP + creatinc phO5pho,crcatine. + ADP. kinase And in the muscle contraction the reaction is rmerse: phöiphocreatinc + ADP —- —— ł:inase. ATP + creatine The reaction is catalysed by kinase. High activities of this ename:are first of all in muscle cells and aho in-,:myomrd. 3.2 The .aiæ of practical exercise Thcaim of laboratory practice is to determine activity of creative kinase in the muscle, thc mjucard and the liver of a rabbit. We compare acti›i in homogenates of these tissues. First of all in the muscle, but also in the myoœrd this enzyme plays important rote ia the #nergetics of cell and its activity in thae tissues ú high. On the contrary the İiver cell does not w•e phosphocreatiae as reserve of ener an4 so aciiviy of weatinc kinase in ib‹e liver tissue is low. The acti iș of creative is also determined in the clinical laboratory. In injury of myocard this cngee is liberated into blood an4 so the determination of ac:i› iij’ of crcz line kinase is one of the basic parameters in diagnosis of the infarct of myocard. 3.3 Practical part 33.1 The determination of phosphocreati.ne Ńnase in tissues of muscle, mș ocard liver of rabbit Creatine ki:nase forms ATP from phosphocreatine and ADP: CK 2" CODH ’H-ûH2" Cß0H in 0,5 0,5 Incubatio:n 15 minutes 0,5 0,0 0;5 0,5 0,5 0o 0,5 0,5 0,5 0,5 Samples let siand. for i0 minutes Physiologica1›alam ofcreatime kinase in serious tissues are very different. For insJance,in the liver activity o( creatine kinase is only 12 nka,t/g fresh tissue, in the myocard is activity about 6ykai/g tiss,ue an,d!:in the muscle 33 ¿i kai/g1issue. If we compare activity in the liver 8 ñd i.n the muscle, hero is activity 21 3.4.2 The ev.aIuation From obtained absorbance values of ’arious tissues we read the amount of created crmiine in the react ion from the analytical cun’e. Then from these values we calculate the amount pcr 1 C of fresh iissue. Actin ity of crcaiine kinase c express in nkat or kat per gram of fresh tissue. Illustrate we acti âiy of enp’mc in column graphs and determinc how many time is the acti icy of creai ine kinase higher in the muscle tissue than in the liver and the m ocurd.