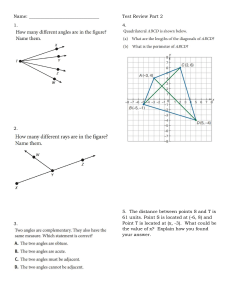
Materials Science HW: Crystal Structures, Density, Indices
advertisement
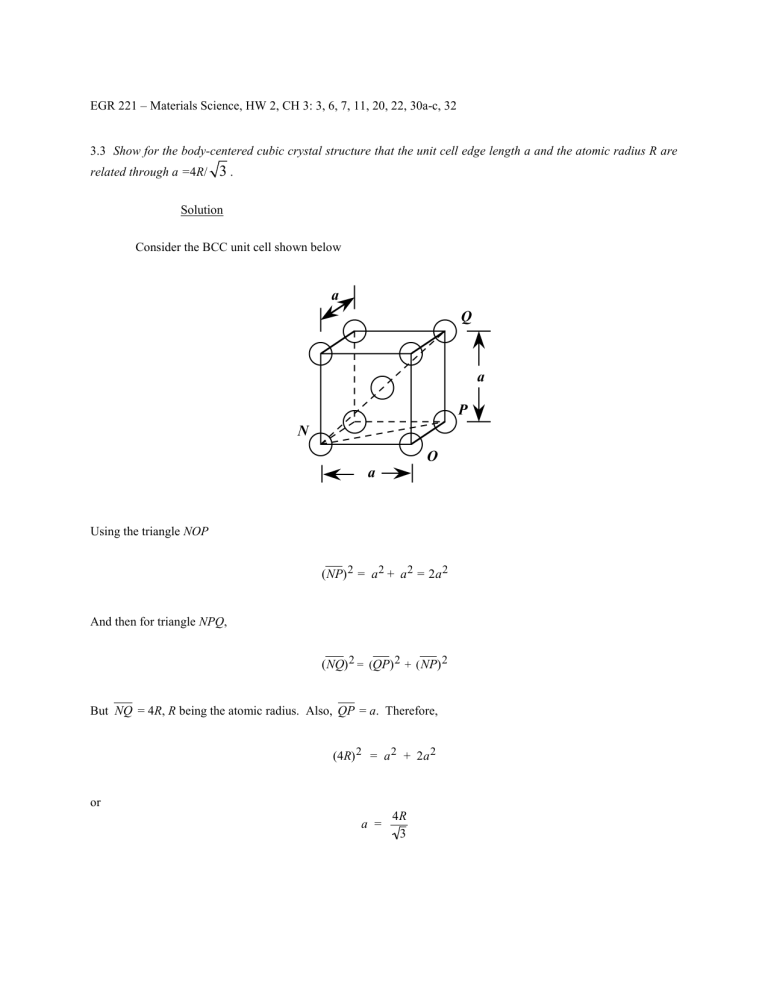
EGR 221 – Materials Science, HW 2, CH 3: 3, 6, 7, 11, 20, 22, 30a-c, 32 3.3 Show for the body-centered cubic crystal structure that the unit cell edge length a and the atomic radius R are related through a =4R/ 3. Solution Consider the BCC unit cell shown below Using the triangle NOP (NP) 2 = a 2 + a 2 = 2a 2 And then for triangle NPQ, (NQ) 2 = (QP) 2 + ( NP) 2 But NQ = 4R, R being the atomic radius. Also, QP = a. Therefore, (4R) 2 = a 2 + 2a 2 or a = 4R 3 3.6 Show that the atomic packing factor for HCP is 0.74. Solution The APF is just the total sphere volume-unit cell volume ratio. For HCP, there are the equivalent of six spheres per unit cell, and thus 4π R 3 = 8π R 3 VS = 6 3 Now, the unit cell volume is just the product of the base area times the cell height, c. This base area is just three times the area of the parallelepiped ACDE shown below. The area of ACDE is just the length of CD times the height BC . But CD is just a or 2R, and BC = 2R cos (30°) = 2R 3 2 Thus, the base area is just 2 R 3 2 AREA = (3)(CD)(BC) = (3)(2 R) = 6R 3 2 and since c = 1.633a = 2R(1.633) VC = (AREA)(c) = 6 R 2 c 3 (3.S1) = (6 R 2 3 ) (2)(1.633)R = 12 3 (1.633) R 3 Thus, APF = VS VC 8π R 3 = 12 3 (1.633) R 3 = 0.74 Density Computations 3.7 Iron has a BCC crystal structure, an atomic radius of 0.124 nm, and an atomic weight of 55.85 g/mol. Compute and compare its theoretical density with the experimental value found inside the front cover. Solution This problem calls for a computation of the density of iron. According to Equation 3.5 ρ = nAFe VC N A For BCC, n = 2 atoms/unit cell, and 4 R 3 VC = 3 Thus, ρ = = nAFe 4 R 3 NA 3 (2 atoms/unit cell)(55.85 g/mol) [(4) (0.124 × 10-7 cm) / 3] /(unit cell) (6.022 × 1023 atoms/mol) 3 = 7.90 g/cm3 The value given inside the front cover is 7.87 g/cm3. 3.11 Zirconium has an HCP crystal structure and a density of 6.51 g/cm3. (a) What is the volume of its unit cell in cubic meters? (b) If the c/a ratio is 1.593, compute the values of c and a. Solution (a) The volume of the Zr unit cell may be computed using Equation 3.5 as VC = nAZr ρN A Now, for HCP, n = 6 atoms/unit cell, and for Zr, AZr = 91.22 g/mol. Thus, VC = (6 atoms/unit cell)(91.22 g/mol) (6.51 g/cm3)(6.022 × 10 23 atoms/mol) = 1.396 × 10-22 cm3/unit cell = 1.396 × 10-28 m3/unit cell (b) From Equation 3.S1 of the solution to Problem 3.6, for HCP VC = 6 R 2 c 3 But, since a = 2R, (i.e., R = a/2) then a 2 3 3 a2 c VC = 6 c 3 = 2 2 but, since c = 1.593a VC = 3 3 (1.593) a 3 = 1.396 × 10 -22 cm3/unit cell 2 Now, solving for a (2)(1.396 × 10 -22 cm3 ) 1/3 a = (3) ( 3) (1.593) = 3.23 × 10-8 cm = 0.323 nm And finally c = 1.593a = (1.593)(0.323 nm) = 0.515 nm Crystal Systems 3.20 Below is a unit cell for a hypothetical metal. (a) To which crystal system does this unit cell belong? (b) What would this crystal structure be called? (c) Calculate the density of the material, given that its atomic weight is 141 g/mol. Solution (a) The unit cell shown in the problem statement belongs to the tetragonal crystal system since a = b = 0.30 nm, c = 0.40 nm, and α = β = γ = 90°. (b) The crystal structure would be called body-centered tetragonal. (c) As with BCC, n = 2 atoms/unit cell. Also, for this unit cell VC = (3.0 × 10−8 cm) 2 ( 4.0 × 10−8 cm) = 3.60 × 10−23 cm3/unit cell Thus, using Equation 3.5, the density is equal to ρ = nA VC N A = (3.60 × (2 atoms/unit cell) (141 g/mol) cm3/unit cell)(6.022 × 10 23 atoms/mol) 10 -23 = 13.0 g/cm3 Point Coordinates 3.22 List the point coordinates for all atoms that are associated with the FCC unit cell (Figure 3.1). Solution From Figure 3.1b, the atom located of the origin of the unit cell has the coordinates 000. Coordinates for other atoms in the bottom face are 100, 110, 010, and 1 1 2 2 0. (The z coordinate for all these points is zero.) For the top unit cell face, the coordinates are 001, 101, 111, 011, and 11 22 1. Coordinates for those atoms that are positioned at the centers of both side faces, and centers of both front and back faces need to be specified. For the front and back-center face atoms, the coordinates are 1 respectively. While for the left and right side center-face atoms, the respective coordinates are 3.30 Within a cubic unit cell, sketch the following directions: (a) [1 10], (b) [1 2 1], (c) [01 2], Solution The directions asked for are indicated in the cubic unit cells shown below. 1 2 0 1 2 11 22 and and 0 1 1 1 . 2 2 1 1 2 2 , 3.32 Determine the indices for the directions shown in the following cubic unit cell: Solution Direction A is a [ 430] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system x y 2a b 3 2 2 1 3 2 Projections – Projections in terms of a, b, and c – z 0c 0 Reduction to integers –4 Enclosure 3 0 [ 430] Direction B is a [23 2] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system x Projections Projections in terms of a, b, and c Reduction to integers 2a 3 2 3 2 Enclosure y –b –1 –3 z 2c 3 2 3 2 [23 2] Direction C is a [13 3 ] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system x Projections Projections in terms of a, b, and c Reduction to integers a 3 1 3 1 Enclosure y z –b –c –1 –1 –3 –3 [13 3 ] Direction D is a [136 ] direction, which determination is summarized as follows. We first of all position the origin of the coordinate system at the tail of the direction vector; then in terms of this new coordinate system Projections Projections in terms of a, b, and c Reduction to integers Enclosure x y a b 6 1 2 1 6 2 1 3 [136 ] z –c –1 –6