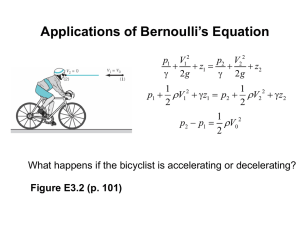
Principles of Energy Conversion NAVEEN H E Department of Mechanical Engineering UNIT-5 Principles of Turbomachinery Class-2: Thermodynamic Analysis NAVEEN H E Department of Mechanical Engineering Principles of Energy Conversion Class 2 : Contents • Static and Stagnation states • Applications of 1st law of TD to turbomachines • Applications of 2nd law of TD to turbomachines Principles of Energy Conversion Static and Stagnation States Static state: Fluid at rest is said to be in a static state properties measured of fluid at rest are called static properties Note: In a turbo machine we only deal with moving fluid. Principles of Energy Conversion Static and Stagnation States The Stagnation or Total State: The stagnation state is defined as the terminal state of a fictitious, isentropic, work-free and steady-flow process during which the macroscopic kinetic and potential energies of the fluid particle are reduced to zero, the initial state for the process being the static state. Note: Even though stagnation state can never be achieved by any real process, it is very important concept in the analysis of turbo machine Pitot static tube Wall pressure tap Principles of Energy Conversion Application of 1st law of TD 1st law of Thermodynamics for a steady flow machine and determination of stagnation properties (V2 V1 ) q w (h2 h1 ) g ( z2 z1 ) 2 2 2 Applying this for the analysis of turbo machine, we arrive at stagnation state. We may write, (V02 V 2 ) q w (h0 h) g ( z0 z) 2 Where the subscript ‘0’ refers to the stagnation state. Hence, we have h0, V0, z0 are the stagnation properties, h, V, and z are the static properties. Principles of Energy Conversion Application of 1st law of TD But we know that in this process q 0, w 0, V0 0, gz 0 0 V2 0 h0 h gz 2 V2 Thus stagnation enthalpy h0 h gz 2 Stagnation entropy s0 s Since the process is isentropic Principles of Energy Conversion Application of 1st law of TD For a closed system Tds dh vdp ds 0 There fore, dh vdp p0 h0 h vdp p r.h.s.may be integrated only if the relationship between ‘v’&’p’ is known. Principles of Energy Conversion Application of 1st law of TD h0 h v( p 0 p ) For an incompressible fluid, The term v 2 2 p 0 p (h0 h) p 0 p (h0 h) V2 p 0 p [( h gz ) h] 2 is the pressure equivalent of velocity. ‘ρgz’ which is the pressure equivalent of height can be neglected since it is very small compared to other terms. 2 Hence we can write, Stagnation pressure p p v 0 2 According to first law of T.D , the stagnation enthalpy and stagnation pressure should be constant along any streamline which experiences no energy transfer as heat or as work. Principles of Energy Conversion Application of 1st law of TD For a change from static to stagnation state of an incompressible fluid since there is no entropy change and pdv = 0 T.ds = du + p.dv Which yields du = 0. Hence, u = u0, the internal energies of an incompressible fluid in the static and stagnation states are equal. u = u0 cT = cT0 so, T = T0 Principles of Energy Conversion Application of 1st law of TD Perfect gas: We have h0 C PT0 h C PT Substituting in expression for stagnation enthalpy and rearranging V2 gz T0 T 2CP Cp The term gz CP which may be called temperature equivalent of height is negligible compared to other terms and may be neglected. V2 Stagnation temperatu re T0 T 2Cp Principles of Energy Conversion Application of 2nd law of TD Apply the steady flow energy equation for the analysis of turbo machine. V22 V12 q w (h2 h1 ) g ( z 2 z1 ) 2 It is also true that, thermal loses are minimal compared to the amount of work transferred & hence may be neglected. Hence we may write, V22 V12 w (h2 gz 2 ) (h1 gz1 ) 2 2 w (h02 h01 ) Where, h02 & h01 are stagnation exit & entry respectively. - w = ∆h0. In a power generating turbo machine, ∆h0 is negative (since h02 <h01 ) & hence w is positive. for a power absorbing turbo machine, ∆h0 is positive (since h02>h01) &hence w is negative. Principles of Energy Conversion Application of 2nd law of TD From the 2nd law of Thermodynamics: Tds dh vdp dw vdp Tds 2 w vdp Tds 1 In the above relation, we note that vdp would be a negative quantity for a power generating turbo machine & positive for power absorbing turbo machine. Hence Tds which is always a positive quantity would reduce the amount of work generated in the former case & increase the work absorbed in the later case. THANK YOU NAVEEN H E Department of Mechanical Engineering naveenhe@pes.edu