JAN JERICHO MENTOY - Exercise No.10 Reactions and Preparations of Carboxylic Acid and Derivatives
advertisement

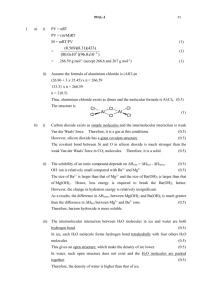
Exercise No. 10 Reactions and Preparations of Carboxylic Acid and Derivatives 1. By means of a suitable reaction, show how each of the ff. compounds can be prepared from propionic acid. More than one step may be required. a. ethyl propionate H+ + CH3 -CH2-OH + H2O ethyl propionate Ethanol Water b. propionyl chloride + SOCl2 + SO2 + HCl Hydrochloric acid propionyl chloride Sulfur dioxide c. N-methyl propionamide + SOCl2 + SO2 + HCl Hydrochloric acid propionyl chloride + H2N-CH2CH3 Sulfur dioxide CH3-CH2-C-NH- CH3 O N-methyl propionamide propionyl chloride d. propionic anhydride + NaOH + HCl Cl propionyl chloride propionic anhydride Hydrochloric acid e. methyl propylamine (CH3NHCH2CH2CH3) + H2N-CH3 NH - CH3 N-methylpropanamide LiAlH4 NH - CH3 H20 methylpropylamine 2. Write equations to show the reaction of propanoyl chloride with: a. Water + HCl + H 2O Hydrochloric acid propionic acid propanoyl chloride b. Ammonia + NH3 + NH4Cl NH2 Ammonium chloride propionamide / propanamide c. Aniline O NH-C-CH2-CH3 + + HCl N-phenylpropanamide Hydrochloric acid d) sodium acetate + + NaCl O Sodium chloride acetic propanoic anhydride d. isopropyl alcohol CH-CH3 + CH3 + HCl Hydrochloric acid isopropyl propanoate / isopropyl propionate f. benzene + AlCl3 + + HCl AlCl3 Hydrochloric acid propiophenone e. dimethylamine (CH3-NH-CH3) + + HCl Hydrochloric acid N,N-dimethylpropionamide 3. By means of a series of equations outline a synthesis for each of the ff. compounds from the indicated starting material. You may use any other reagents needed. a. ethyl butanoate from n-butyl alcohol KMnO4 n-butyl alcohol butanoic acid H2SO4 + ethyl butanoate ethanol butanoic acid b. propionyl chloride from n-propyl alcohol KMnO4 + + PCl3 n-propyl alcohol propanoic acid propionyl chloride c. benzamide from toluene + SOCl2 toluene benzoyl chloride benzoic acid NH3 benzoyl chloride benzamide H3PO4 phosphoric acid d. sec-butyl acetate from 2-butene O O3 CH3-CH=CH-CH3 CH3-CH O CH-CH3 (CH3)2S + O acetaldehyde H2CrO4 H2SO4 + sec-butyl acetate 2-butanol acetic acid acetaldehyde e. n-butyric anhydride from n-butyl alcohol KMnO4 n-butyl alcohol NaOH + Butanoic acid Butanoic acid butanoyl chloride n-butyric anhydride 4. Predict the products (if any) of the reaction of each of the ff. reagents with benzoic acid. a. NaOH + NaOH O-Na+ + H20 water sodium benzoate b. LiAlH4 + LiAlH4 benzoyl alcohol c. PCl5 + + PCl5 benzoyl chloride HCl + POCl 3 hydrochloric acid phosphorus oxychloride d. NaBH4 + NO REACTION NaBH4 e. CH3OH + O-CH3 CH3OH + H2O water methyl benzoate f. H2, Pt, 1 atm, room temp. H2 NO REACTION Pt, 1 atm g. SOCl2 + + SOCl2 SO2 + HCl sulfur dioxide benzoyl chloride h. hydrochloric acid conc. HCl conc. HCl NO REACTION 5. Write the products of the following reaction sequences. a) C6H5-CH3 i. Br2, light Br2, light C6H5-CH3 ii. + HBr NaCN NaCN C6H5-CH2Br iii. C6H5-CH2Br benzyl bromide benzyl cyanide C6H5-CH2CN sodium bromide + NaBr H2O, heat H2O,heat C6H5-CH2CN - C6H5-CH2-C- O NH4 + O ammonium benzyl enolate iv. NaOH NaOH - C6H5-CH2-C- O Na + C6H5-CH2CN + NH3 + H2O O sodium benzyl enolate b) ammonia water OH H+, heat i. OH H+ + H2O heat water cylohexene ii. O3 O CH O3 CH O O cylohexene ozonide iii. Zn, H2O O O CH O CH C - CH2- CH2- CH2- CH2 -C Zn O O H H2O H hexanedial iv. H2Cr2O7 O O O H2Cr2O7 O C - CH2- CH2- CH2- CH2 -C C -CH2- CH2- CH2- CH2- C OH H OH H adipic acid / hexanedioic acid c) C6H5-CH2Br i. Mg/ dry ether MgBr Mg Dry ether ii. benzyl magnesium bromide HCHO OH CH2O MgBr C- H H iii. 2-phenylethanol KMnO4 O OH KMnO4 C-H C -OH H phenylacetic acid d) benzene i. CH3I, AlCl3 CH3 AlCl3 + + CH3I HI hydroiodic acid toluene Ii. KMnO4 CH3 KMnO4 benzoic acid ii. SOCl2 SOCl2 + SO2 + HCl sulfur dioxide hydrochloric acid benzoyl chloride iii. benzene, AlCl3 AlCl3 + benzophenone e) CH3CH2CHO i. CH3MgBr OH CH3MgBr CH3 - CH2 -CH -CH3 2-butanol ii. H2Cr2O7 O OH H2Cr2O7 CH3 - CH2 -CH -CH3 CH3 - CH2 -C -CH3 2-butanol 2-butanone iii. I2, base O O CH3 - CH2 -C -CH3 I2 Na+ CH3 - CH2 -C -O Base [NaOH] + CHI3 Iodoform Sodium propionate 6. Write the equations showing the reactions of propanoic acid with: a. thionyl chloride + + SO2 + HCl SOCl2 propionyl chloride b. methanol + + H2O CH3OH O-CH3 methyl propionate c. Ammonia water + NH3 - NH4 + ammonium propionate 7. Write equations to show how each of the ff. compounds could be converted into benzoic acid. a. Bromobenzene MgBr Mg CO2 Dry ether H+ + MgBr(OH) OMgBr dry ice benzoic acid bromobenzene b. Toluene KMnO4 ∆(Heat) benzoic acid toluene c. Benzonitrile + H2O H+ + NH4 benzoic acid benzonitrile d. benzyl alcohol KMnO4 benzyl alcohol benzoic acid 8. Write equations to show each of the ff. compounds could be converted to n-butyric acid. a. n-butyl alcohol KMnO4 N-butyric acid b. n-propyl alcohol Br + H2O + HBr Br Mg MgBr CO2 COOMgBr COOMgBr N-butyric acid H+ 9. Write the equations to show how isobutyric acid could be converted to each of the following using needed reagents a. ethyl isobutyrate H+ + + H2O ethyl isobutyrate b. isobutyryl chloride + PCl3 isobutyric acid + H3PO4 c. Isobutyramide isobutyryl chloride + SO2 + HCl + SOCl2 isobutyric acid NH3 + NH4Cl isobutyryl chloride isobutyramide d. magnesium isobutyrate O-Mg+ + MgCO3 isobutyric acid + CO2 + H2O magnesium isobutyrate e. isobutyl alcohol 4 (CH3)2CHCOOH + 3 LiAlH4 ether + [(CH3)2CHCH3O]4AlLi + 2LiAlO2 + 4H2 H (CH3)2CHCH2OH isobutyl acid isobutyric acid 10. Write the balanced equations, naming all organic products (if any) for the reaction of – butyryl chloride with: a. H2O + HCl + H2O butanoic acid Hydrochloric acid b. isopropyl alcohol + + HCl isopropyl butanoate c. Hydrochloric acid p-nitrophenol + HCl + Hydrochloric acid O NO2 p-nitrophenyl butyrate d. Ammonia + NH4Cl + NH3 Butanamide/ butyramide ammonium chloride e. toluene, AlCl3 + AlCl3 + HCl Hydrochloric acid COCH2CH2CH3 1-(4-methylphenyl)butan-1-one f. nitrobenzene, AlCl3 + HCl AlCl3 + Hydrochloric acid 1-(4-nitrophenyl)-1-butanone 11. An unknown ester C6H12O2 was hydrolyzed with water and acid to produce an acid X and an alcohol Y. Oxidation of Y with chromic acid produced X. What was the structure of the original ester. Write the equations for all the reactions that occur. RCOOCH2R H3O+ RCOOH + R’OH H2CrO4 Ester propyl propanoate H3O+ H2CrO4 propanol (Y) propanoic acid (X)