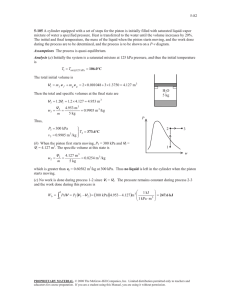
CH 19 revision Indicate the answer choice that best completes the statement or answers the question. 18. A diver is 50 m below the surface of the water. About how many times greater than atmospheric pressure is the water pressure? a. 4.8 b. 20 c. 5.1 d. 0.5 19. Which principle explains why an object seems to weigh less when submerged in water? a. Bernoulli’s principle b. Boyle’s Law c. Archimedes’ principle d. Principle of linear expansion 21. A gas is collected in a 4.24 L container at 0°C. The pressure of the gas is determined to be 137 kPa. Determine the number of moles of gas present. a. 0.256 moles b. 0.0209 moles c. 0.0025 moles d. 5.724 moles 22. The bimetallic strip in a thermostat operates according to which principle? a. Bernoulli’s principle b. Boyle’s Law c. Archimedes’s principle d. Thermal expansion 23. A hydraulic piston is used to lift 1030 N using 45 N of force. If the platform supporting the weight has an area of 1.2 m2, what is the area of the piston that force is applied to? a. 0.036 m2 b. 27.5 m2 c. 19.1 m2 d. 0.052 m2 24. Which is not a unit of pressure? a. N/m2 b. Pa c. kPa d. N/cm 26. What is the buoyant force on a copper cube that measures 0.5 m on each side when it is submerged in water? The density of copper is 8.92 103 kg/m3 and the density of water is 1.00 103 kg/m3. a. 12.3 N b. 820 N c. 4900 N d. 1200 N CH 19 revision 27. Which describes a volatile liquid? a. strong cohesion b. weak cohesion c. strong cohesion and weak adhesion d. does not evaporate quickly 28. Which is true about the forces in a liquid that wets, or spreads into a film on a surface? a. adhesion is greater than cohesion b. cohesion is greater than adhesion c. the two forces are equal d. neither of these forces are acting 29. Small particles, like dust, settle on the top of a liquid rather than sinking. Which term describes this phenomenon? a. adhesion b. volatile c. evaporative cooling d. surface tension 31. A rectangular block with dimensions 4 cm x 4 cm x 10 cm has a weight of 750 N. By what factor is the pressure it exerts increased when it is standing on its smaller end compared to when it is lying on its side? a. 4 times b. 2.5 times c. 18.75 times d. 4.7 times 32. How much pressure is exerted by a force of 600 N spread over a 20 m2 area? a. 30 N b. 2700 Pa c. 30 Pa d. 12000 Pa 33. A sample of gas has a volume of 0.35 m3 and a pressure of 1.42 kPa when it is at 273 K. What will the new pressure be if it is allowed to occupy a volume of 0.63 m3 at a temperature of 340 K? a. 0.63 kPa b. 29000 kPa c. 3.2 kPa d. 0.98 kPa 86. The cross-sectional area of an air piston is m2. When the piston is pushed in a downward direction, the internal pressure is 19.0 kPa more than the atmospheric pressure. Calculate the force with which the piston should be pushed if the atmospheric pressure is 101.3 kPa. a. 7.16 N b. 1.65 N c. 10.5 N d. N 87. A hydraulic stamping machine is used to apply large forces to metal sheets to convert them into various shapes. One such machine has cylindrical pistons of diameters 1.46 cm and 40.0 cm, with a force of 841 N applied to the smaller piston. Calculate the output force that acts on the sheets through the larger piston. a. N b. N c. N d. N CH 19 revision Answer Key 18. a 19. c 21. a 22. d 23. d 24. d 26. d 27. b 28. a 29. d 31. b 32. c 33. d 86. c 87. b CH 19 revision 1. Which state of matter is the most common in the universe? A. solid B. gas C. liquid D. plasma → 2. As water cools below 4°C, what happens? A. it changes to an amorphous solid B. it contracts C. it melts D. it expands → 3. What causes air pressure? A. air particles vaporize B. air particles flow through an object C. air particles hit an object → D. air particles suck away from an object 4. What are the four stages of matter in order from least kinetic energy to most kinetic energy? A. plasma, gas, liquid, solid B. plasma, solid, gas, liquid C. solid, liquid, gas, plasma → D. solid, liquid, plasma, gas 5. What are the particles in plasma? A. free nuclear particles of protons, neutrons, and electrons B. positively charged ions and negatively charged electrons → C. negatively charged ions and positively charged protons D. free neutrons CH 19 revision 6. __________ have no definite shape and flow. A. Crystals B. Solids C. Metals D. Fluids → 7. Pressure is measured as __________. A. FA B. F/A → C. A/F D. F + A 8. A particle is moving so fast in a liquid that it escapes the liquid's cohesive force. This is an example of __________. A. condensation B. sublimation C. evaporation → D. melting 9. Surface tension is a result of __________ in a fluid. A. nuclear forces B. adhesive forces C. cohesive forces → D. kinetic forces 10. __________ is the force that acts between particles of different substances. A. Rehesion B. Cohesion C. Elasticity D. Adhesion → CH 19 revision 11. Which of the following does pressure in water not depend on? A. depth B. density C. shape → D. gravity 12. The buoyant force is in which direction? A. toward higher pressures B. upward → C. circular D. downward 13. In the figure below, if the chunk of steel were cut in half and one of the pieces were placed in the same liquid, how would it behave? A. It would float mostly submerged. B. It would sink to the bottom of the container. → C. There is insufficient information to answer the question. D. It would float almost entirely above the surface. 15. To rise in water, a fish uses its air bladder to __________. A. displace more water → B. increase water pressure C. increase air pressure D. displace less water 16. __________ states that any change in pressure applied to any point on a confined fluid is transmitted undiminished throughout the fluid. A. Boyle's law B. Pascal's principle → C. Galileo's law D. Dalton's law CH 19 revision 17. If you wanted to use a setup like the one in the figure bellow to create an upward force triple that of the downward force you exert, which of the following combination of piston areas could accomplish this? A. A1, 6 m2; A2,10 m2 B. A1, 6 m2; A2, 18 m2 → C. A1, 6 m2; A2, 2 m2 D. A1, 6 m2; A2, 8 m2 18. Why does ice float? A. It is an amorphous solid. B. It has strong cohesive properties. C. It has a lower density than water. → D. It has a higher density than water. 19. Which is an example of Pascal's principle? A. a straw B. hydroplaning wheels C. hydraulic brakes → D. a siphon 20. According to Archimedes' principle, an object immersed in fluid has an upward force on it equal to __________. A. the weight of the fluid displaced → B. the weight of all the fluid in the container C. the weight of the fluid displaced minus the weight of the object D. the weight of the object