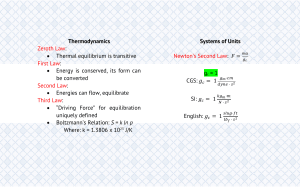
Prepared by: EFREN A. DELA CRUZ E-mail Address: eadelacruz@clsu.edu.ph Central Luzon State University Science City of Muñoz 3120 Nueva Ecija, Philippines Instructional Module for the Course (MENGR 3100 – Basic Mechanical Engineering) Module 1 Fundamental Concepts and Definitions Overview Every science has its own foundation and unique terminologies, as in the course of thermodynamics and heat transfer. Accurate definition relating to basic concepts makes a sound foundation for the development of a science and prevents possible confusions. The goal of this topic is to introduce some fundamental concepts and definitions used in the study of engineering thermodynamics and heat transfer. In most instances the discussions were brief, and further elaboration is provided in the succeeding modules illustrations and sample problems were also provided for clearer understanding of the course. MENGR 3100 – Basic Mechanical Engineering I. Objectives Upon successful completion of the module, students are expected to: a. Explain some of the basic concepts of the course. b. Define or recognize the terminologies used in thermodynamics and heat transfer. c. Solve property related problems with proper units and dimensions. II. Learning Activities 1 Basic Principle, Concepts and Definitions The word thermodynamics was derived from Greek words “Therme” means heat and “Dynamis” means power. Thermodynamics is the science that deals with various phenomena of energy and the related properties of matter, especially of the laws of transformation of heat into other forms of energy. Thermodynamics Substance - a fluid that receives, transports, and transforms energy. 1.1 Thermodynamics System An important step in any engineering analysis is to describe precisely what is being studied. A system is defined as a quantity of matter or a region in space chosen for study. It may be as simple as a closed, rigid-walled tank or as complex as an entire Industrial refinery. The composition of the matter inside the system may be fixed or may be changing through chemical or nuclear reactions. The shape or volume of the system being analyzed is not necessarily constant, as when a gas in a cylinder is compressed by a piston or an inflated balloon. The mass or region outside the system is called the surroundings. The real or imaginary surface that separates the system from its surroundings is called the boundary (Fig. 1.1). The boundary of a system can be fixed or movable. Note that the boundary is the contact surface shared by both the system and the surroundings. Mathematically speaking, the boundary has zero thickness, and thus it can neither contain any mass nor occupy any volume in space. Fig. 1.1 System, Surroundings and Boundary Page 2 of 16 MENGR 3100 – Basic Mechanical Engineering Types of Systems Two basic kinds of systems are studied in this material. Systems may be considered to be closed or open, depending on whether a fixed mass or a fixed volume in space is chosen for study. A closed system (also known as a control mass or just system when the context makes it clear) consists of a fixed amount of mass, and no mass can cross its boundary. That is, no mass can enter or leave a closed system, as shown in Fig. 1.2 (a). But energy, in the form of heat or work, can cross the boundary; and the volume of a closed system does not have to be fixed. If, as a special case, even energy is not allowed to cross the boundary, that system is called isolated system. Consider the piston-cylinder device shown in Fig. 1.2 (b). Let us say that we would like to find out what happens to the enclosed gas when it is heated. Since we are focusing our attention on the gas, it is our system. The inner surfaces of the piston and the cylinder form the boundary, and since no mass is crossing this boundary, it is a closed system. Notice that energy may cross the boundary, and part of the boundary (the inner surface of the piston, in this case) may move. Everything outside the gas, including the piston and the cylinder, is the surroundings. (a) (b) Fig. 1.2 Closed System An open system, or a control volume, as it is often called, is a properly selected region in space. It usually encloses a device that involves mass flow such as a compressor, turbine, or nozzle Fig. 1.3. Flow through these devices is best studied by selecting the region within the device as the control volume. Both mass and energy can cross the boundary of a control volume. min mout Fig. 1.3 Open System Page 3 of 16 MENGR 3100 – Basic Mechanical Engineering Thermodynamics deals with the macroscopic as opposed to microscopic analysis in describing the system behavior. In microscopic analysis we must look and analyze the molecular action. In macroscopic we can define the system behavior with the overall effect of the molecular interaction. 1.2 Properties of Thermodynamics substance/ system. Characteristic quality of the entire systems and depends not on how the system change state but only on the final particular state of the substance/system. Extensive Properties - those properties that vary directly with mass. Ex. mass, volume of solids. Intensive properties -Those properties that are independent of the mass. Ex. Tem. , Pressure. Extensive properties per unit mass (specific properties) are considered as intensive properties Ex. Specific volume. 1.3 Phase and state of Thermodynamic substance. Phase- quantity of matter that is homogeneous throughout (solid, liquid, gas) Change of Phase Solid to Liquid Liquid to Solid Liquid to Gas Gas to liquid Solid to Gas - Melting - Solidification (Freezing) - Evaporation - Condensation - Sublimation State of Substance - condition of a substance as described by certain observable macroscopic parameter called Properties. 1.4 Processes and Cycles Process- Simply the change of state Isothermal Isobaric Isometric Adiabatic Isenthalpic Polytropic Types of processes - constant temperature. - constant pressure - constant volume - constant entropy (PVk = C) - constant enthalpy – PVn = C Cycle - a series of processes one after the other such that the initial and final states are the same. Page 4 of 16 MENGR 3100 – Basic Mechanical Engineering 1.5 Dimensions and System of Units When engineering calculations are performed, it is necessary to be concerned with the units of the physical quantities involved. A unit is any specified amount of a quantity by comparison with which any other quantity of the same kind is measured. For example, meters, kilometers, feet, and inches are all units of length. Seconds, minutes, and hours are alternative time units. SI Units SI is the abbreviation for Système International d’Unités (International System of Units), which is the legally accepted system in most countries. The SI base units for mass, length, and time are listed in Table 1.1. They are, respectively, the kilogram (kg), meter (m), and second (s). The SI unit of force, called the newton, is defined in terms of the base units for mass, length, and time. Newton’s second law of motion states that the net force acting on a body is proportional to the product of the mass and the acceleration, written F α ma. The newton is defined so that the proportionality constant in the expression is equal to unity. F = kma or k = ma/F Where k is proportionality constant Table 1.1 SI Units for Mass, Length, Time and Force Quantity mass length time force Unit kilogram meter second newton (= 1 kg m/s2) Symbol kg m s N Other Units Although SI units are the worldwide standard, at the present time many segments of the engineering community regularly use some other units. A large portion of market stock of tools and industrial machines and much valuable engineering data utilize units other than SI units. For many years to come, engineers will have to be conversant with a variety of units. Accordingly, in this section we consider the alternative units for mass, length, time, and force listed in Table 1.2. Table 1.2 OtherUnits for Mass, Length, Time and Force Quantity Unit Symbol mass pound mass lb slug slug length foot ft time second s force pound force lbf (= 32.174lb ft/s2 = 1 slug ft/s2) Page 5 of 16 MENGR 3100 – Basic Mechanical Engineering SI is based on a decimal relationship between units. The prefixes used to express the multiples of the various units are listed in Table 1.3. They are standard for all units, and the student is encouraged to memorize them because of their widespread use. Table 1.3 Standard Prefixes in SI Units Factor Prefix Symbol 24 10 yotta Y 1021 zetta Z 18 10 exa E 1015 peta P 1012 tera T 9 10 giga G 106 mega M 3 10 kilo k 102 hecto h 1 10 deka da 10-1 deci d -2 10 centi c -3 10 milli m 10-6 micro μ -9 10 nano n 10-12 pico p -15 10 femto f 10-18 atto a -21 10 zepto z 10-24 yocto y System of units where k is unity but not dimensionless. CGS System: 1-dyne force accelerates 1-gram mass at 1 cm./s2 & SI: 1 Newton force accelerates 1 kg. mass at 1 m/s2 English: 1 lb force accelerate 1 slug mass at 1 ft/s2 For CGS o k = 1gm.cm/dyne. s2 For SI Or MKS: o k = 1 kgm.m/Newton.s2 For English: o k = 1slug.ft/lbf. s2 Page 6 of 16 MENGR 3100 – Basic Mechanical Engineering System of units where k is neither unity nor dimensionless. If the same word were used for both mass and force in a given system CGS System: 1-gram force accelerates 1-gram mass at 980.665 cm./s2 MKS: 1-kg force accelerates 1 kg- mass at 9.80665 m/s2 English: 1-lb force accelerate 1 lb-mass at 32.174 ft/s2 For CGS o k = 980.665 gm.cm/gf. s2 For SI Or MKS: o k = 9.80665 kgm.m/kgf.s2 For English: o k = 32.174 lbm.ft/lbf. s2 1.6 Properties and Property Relations Mass And Weight The mass of a body is the absolute quantity of matter in it. Mass equivalent based on gram: Gram- 1 g lb- 454 g kg- 1000 g slug- 14600 g The weight of the body is the force exerted by gravity on the given mass. Relation of Mass and Weight Fg = mg/k from f = ma/k Or W= mg/k Where: W- weight m- mass g- Acceleration due to gravity If observe gravity (go) is not given, then, used the standard gravity (gs) 32.174 ft/s2 32.2-ft/s2 9.80665 m/s2 9.81 m/s2 Page 7 of 16 MENGR 3100 – Basic Mechanical Engineering Other units related to mass and weight For English & MKS W= m go /gs Only for kgf & lbf kgm & lbm Where; go - observe gravity gs - standard gravity If go = gs (m = w) for: 1lbm= 1 lbf & 1kgm= 1kgf Mass Fundamentals Newton’s Physics- mass is constant anywhere in the universe. Law of conservation of mass states that mass is indestructible, provided that there is no nuclear process involved. min= m change + mout And if Δm= O Then min= mout Density, Specific Volume, and Specific Weight Density (ρ)of any substance is its mass per unit volume. ρ= m/V Where: V- Volume occupied by matter Unit of Density English lbm/ft3 lbm/Gal MKS kgm/m3 kgm/l SI kgm/m3 Page 8 of 16 MENGR 3100 – Basic Mechanical Engineering Density of Water at Standard Condition English 62.4 lbm/ft3 8.33 lbm/Gal MKS 1000 kgm/m3 1 kgm/l SI 1000 kgm/m3 Specific Volume is the volume of substance per unit mass v = V/m = 1/(m/V) = 1/ρ For water non at standard condition look @ steam table Specific weight (γ) of any substance is the force of gravity (weight) per unit volume. γ = W/V Units of γ English lbf/ft3 lbf/Gal MKS kgf/m3 kgf/l SI N/m3 For water at standard condition English γ = 62.4 lbf/ft3 = 8.33 lbf/Gal MKS γ = 1000 kgf/m3 = 1 kgf/l SI γ = 9806.65 N/m3 = 9.80665 KN/m3 Page 9 of 16 MENGR 3100 – Basic Mechanical Engineering Ex.1 Two liquids of different densities (ρ1 = 1500 kgf/m3 and ρ2 = 500 kgf/m3) are poured together into 100 L tank, filling it. If the resulting density of the mixture is 800 kgf/m3 find the respective quantities of liquids used. Also, find the weight of the mixture. Solution. For the mass of mixture mm = ρmVm = (800 kg/m3)(0.1m3) = 80 kg mm = m1 + m2 = ρ1V1 + ρ2V2 80kg = 1500 V1 + 500 V2 Fr: (1) V1 + V2 = 0.1 V1 = 0.1 - V2 (2) Substituting (2) to (1) 80 kg = 1500(0.1 – V2) + 500 V2 Or (1500 – 500)V2 = 150 – 80 V2 = 70 kg / (1000 kg/m3) V2 = 0.07 m3 And V1 = 0.03 m3 Also m1 = ρ1V1 = 1500 kg/m3 (0.03 m3) = 45 kg m2 = ρ2V2 = 500 kg/m3 (0.07 m3) = 35 kg and Wm = mm gs = 80 kg (9.81 m/s2) = 784.80 N Page 10 of 16 MENGR 3100 – Basic Mechanical Engineering For fluid passing through a given section (Applying the law of conservation of mass) Q = AV ṁ = Q/v = AV/v = AVρ Where: Q - volume flow rate A - cross sectional area of section V - average speed (velocity) ṁ – mass flow rate ṁin = ṁout AVρin = AVρout Problem to solve Two gaseous streams enter a combining tube and leave as a single mixture. These data apply at the entrance section: A1 = 75 in2, V1 = 500 fps, v1 = 10 ft3/lb, A2 = 50 in2, ṁ 2 = 16.67 lb/s, ρ2 = 0.12 lb/ft3 At exit, V3 = 350 fps, and v3 = 7 ft3/lb. Find the speed at section 2, the flow rate and area at section 3. Sol’n. Page 11 of 16 MENGR 3100 – Basic Mechanical Engineering Specific Gravity Or Relative Density For solids and liquids Ratio of weight to the weight of water of equal volume SG = W/Wwater = γ V/ γwater Vwater = γ / γwater = ρ.g/ ρwaterg = ρ / ρwater = vwater/ v For Gasses SG = MW/MWa Where MWa = 28.97 = 29 Temperature Measures of the degree of hotness or coldness of a body. Always use absolute temp. in the analysis of thermodynamics. For absolute temp. T = 460 + oF in oR = 273 + oC in oK Conversion of oF & oC o F = 1.8 oC + 32 o C = (oF- 32)/1.8 Problem to solve Determine the temperature for which a thermometer with degrees Fahrenheit is numerically twice the reading of the temperature in degrees Celsius. Page 12 of 16 MENGR 3100 – Basic Mechanical Engineering Pressure For solids P = F/A (Stress) For liquids and gasses Measuring pressure 1) Using manometers open to atmosphere a) Absolute press. is greater than atmospheric press. P= Patm + γ h b) Absolute press. is less than atmospheric press. P = Patm - γ h Where P- Absolute press. Patm- atmospheric press. γ - specific weight of fluid in the manometer h- height of fluid 2) Using pressure gages a) absolute press. is greater than atmospheric P = Patm + Pg b) Absolute press. is less than atmospheric P= Patm - Pg Where Pg- gage press. Patm or 1atm =101.325 kPa =14.7 Psi = 760 mm Hg = 29.92 in Hg Problem to solve A vertical column of water will be supported to what height by standard atmospheric pressure? Page 13 of 16 MENGR 3100 – Basic Mechanical Engineering 1.7 Laws of Thermodynamics First Law – also known as the Conservation of Energy principle, states that energy can neither be created not destroyed, it can only change forms. Second Law - deals with the quality of energy (energy degradation). There are two classical statements of this law: Kelvin-Planck statement: It is impossible to construct a device that will operate in a cycle and produce no effect other than the raising of a weight and the exchange of heat with a single reservoir. Clausius statement: It is impossible to construct a device that operates in a cycle and produces no effect other than the transfer of heat from a coolerbody to a hotter body. Third law – states that the entropy of a perfect crystal is zero at the absolute zero of temperature. Zeroth law - when two bodies have equality of temperature with a third body, they in turn have equality of temperature with each other. III. Assessment I. Identification: Read the following statements carefully and try to identify the answer for each item. Write your answer on the space provided before each number. _________________________ 1. Fluid that receives, transports, and transforms energy. _________________________ 2. Quantity of matter with fixed mass and identity in which attention is focused for study. _________________________ 3. Also known as control volume. _________________________ 4. Change on a system state, if a system undergoes from one equilibrium state to another. _________________________ 5. Characteristic quality of the entire systems/substance. Page 14 of 16 MENGR 3100 – Basic Mechanical Engineering II. Problem Solving – Clearly and neatly solve each problem in a separate sheet/s of short bond paper. No solutions and/or units will be considered incorrect. 1. A pump discharges into a 3-m-per-side cubical tank. The flow rate is 300 liters/min, and the fluid has a density 1.2 times that of water. Determine (a) the flow rate in kg/s; (b) the time it takes to fill the tank. 2. A new temperature scale is desired with freezing of water at 100 X and boiling occurring at 1000 X in atmospheric pressure. Derive a conversion between degrees Celsius and degrees X. What is absolute zero in degrees X? 0 0 3. A 3 ft high 4.653 ft diameter cylindrical tank contains 3 lbs. of gas at 80 F and gage pressure of 47 in Hg. (a) What is the temperature in C, R, and K? (b) What is the gas specific volume and density? (c) What is the absolute pressure in Psi and in kPa.? o o o o 4. A pressure cooker operates by cooking food at a higher pressure and temperature than is possible at atmospheric conditions. Steam is contained in the sealed pot, with a small vent hole in the middle of the cover; allowing steam to escape. The pressure is regulated by covering the vent hole with a small weight, which is displaced slightly by the escaping steam. Atmospheric pressure is 100 kPa, the vent hole area is 7 mm , and the Pressure inside should be 250 kPa. What is the mass of the weight? 2 5. A cylindrical tank is 50 in. long, has a diameter of 16 in., and contains 1.65 lb of water. Calculate the specific volume and density of the water. m III. References Moran, M. J. and Shapiro, H. N. 2006. Fundamentals of Engineering Thermodynamics 5th edition. SI version. John Willey & Sons. England. Cengel, Yunus A. 2008. Introduction to Thermodynamics and Heat Transfer. McGrawHill Inc. New York. Moran et al. 2003. Introduction to Thermal Systems Engineering: Thermodynamics, Fluid Mechanics, and Heat Transfer. John Wiley & Sons, Inc. New York Page 15 of 16 MENGR 3100 – Basic Mechanical Engineering Burghardt, M. D. and Harvbach, J. A. 1993. Engineering Thermodynamics 4rth edition. Harper Collins. New York. Thermodynamics Online references and lectures (Yale Open courseware etc..) Page 16 of 16