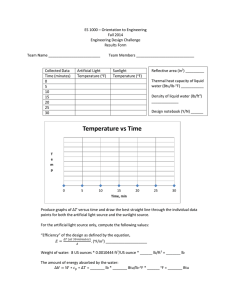
Thermodynamics Zeroth Law: • Thermal equilibrium is transitive First Law: • Energy is conserved, its form can be converted Second Law: • Energies can flow, equilibrate Third Law: • “Driving Force” for equilibration uniquely defined • Boltzmann’s Relation: S = k ln p Where: k = 1.3806 x 1023 J/K Systems of Units Newton’s Second Law: 𝐹 = gc = 1 %+ ⋅(# CGS: 𝑔( = 1 -./0 ⋅ 12 SI: 𝑔( = 1 3%+ ⋅# 4 ⋅ 12 156%⋅78 English: 𝑔( = 1 59 : ⋅ 12 #$ %& gc ¹ 1 % ⋅(# CGS: 𝑔( = 980.66 %+ ⋅ 12 Weight: 𝐹 = #%P %& : 3% ⋅# SI: 𝑔( = 9.8066 3% +⋅ 12 : 59 ⋅78 English: 𝑔( = 32.174 59 +⋅ 12 At the earth’s sea level, the local acc. due to the gravity (𝑔Q ) is: 980.66 cm/ s2 : 9.8066 m/s2 Poundal 𝑙𝑏# ⋅ 𝑓𝑡 𝑔( = 1 𝑝𝑜𝑢𝑛𝑑𝑎𝑙 ⋅ 𝑠 O 32.174 ft/s2 Conversion Temperature 1 slug = 32.174 lbm Celsius à Fahrenheit 1 kgm = 2.205 lbm t(°C) = S [t(°F) – 32] 1 ft = 0.3048 m = 30.48 cm Celsius à Kelvin 1 N = 100,000 dynes t(K) = t(°C) + 273 1 lbf = 32.174 poundal = 4.4484 N Fahrenheit à Rankine 1 kgf = 9.8066 N t(R) = t(°F) + 460 1 lbm = 453.592 gm R Density ρ= # T Mass density of water at sea level at standard condition (Standard temperature of 39.2°F or 4°C and standard pressure of 1 atm) 62.4 lbm / ft3 Specific Volume 𝑣= T # V =W 1000 kgm / m3 1 kgm / L Mass density of air at sea level at standard conditions (Standard temperature of 70°F or 21.11°C and standard pressure of 1 atm) Conversion for Volume 0.075 lbm / ft3 1 gal = 3.78 L 1.2 kgm / m3 1 ft3 = 7.48 gal 1 m3 = 1000 L Specific Weight 𝛾= 𝐹% 𝑚𝑔Q 𝜌𝑔Q 𝑔Q = = = 𝑉 𝑔( 𝑉 𝑔( 𝑔( 𝜐 Specific weight of water at sea level at standard conditions (Standard temperature of 39.2°F or 4°C and standard pressure of 1 atm) 62.4 lbf / ft3 1000 kgf / m3 9.8066 kN / m3 0.98066 dyne / cm3 Specific weight of water at sea level at standard conditions (Standard temperature of 70°F or 21.11°C and standard pressure of 1 atm) 0.075 lbm / ft3 1.2 kgm / m3 11.768 N / m3 Specific gravity of liquids 𝑠. 𝑔 = 𝜌 𝜌] Specific gravity of gases 𝑠. 𝑔 = 𝜌 𝜌$ Specific gravities of common liquids Water – 1.0 Mercury – 13.6 Oil – 0.8 Seawater – 1.03 Measuring pressure Standard atmospheric pressure at sea level Atmospheric Pressure (Barometer) 1 atm 𝑃$8# 𝜌𝑔Q ℎ$8# = 𝑔( Gage Pressure (Manometer) 𝜌𝑔Q ℎ% 𝑃% = 𝑔( 101.325 𝐾𝑃𝑎$ 760 mmHg 33.9 ft 𝐻O 𝑂 14.696 𝑝𝑠𝑖$ 1.0332 kgf / cm2 29.92 in Hg Vacuum 𝜌𝑔Q ℎ% 𝑃% = − 𝑔( 760 torrs 1.01325 bars Internal Energy Terms U = f (m, T) 1 BTU: amount of heat needed to raise the temperature of 1 lbm of water at 68°F by 1°F 𝑢= 𝑈 𝑚 Conversion 1 BTU = 1.055 kJ = 778 ft-lbf 1 kCal = 4.187 kJ = 428.1 kg-m 1 calorie: amount of heat needed to raise the temperature of 1g of water at 14.5°C by 1°C Thermodynamic Property Intensive Property: independent of the mass of the substance Extensive Property: dependent on the mass of the substance Law of Conservation of Mass E = mc2 Where c = speed of light in a vacuum = 2.9979 x 108 m/s Volume flow rate (𝑉̇) 𝑉̇ = A𝜐 Mass flow rate (𝑚̇) 𝑚̇ = Ṫ g = 𝜌𝐴𝜐 Where: A = cross sectional area of the stream 𝜐 = average speed Forms of Energy Potential Energy (PE) à f (m, z) Δ𝑃𝐸 = 𝑚̇ V = 𝑚̇O 𝐴V 𝜐V 𝜌V = 𝐴O 𝜐O 𝜌O 𝑚𝑔Q (𝑧O − 𝑧V ) 𝑔( Kinetic Energy (KE) à f(m, 𝜐) Law of Conservation of Energy Δ𝐾𝐸 = V# O %& (𝜐OO − 𝜐VO ) Internal Energy (U) à f(m, T) ∆𝑈 = 𝑚𝑐p (𝑇O − 𝑇V ) Flow work/ energy (Wf) à f (P, V) ∆𝑊7 = (𝑃O 𝑉O − 𝑃V 𝑉V ) Where: 𝑐p = specific heat at constant volume Enthalpy (H) H = U + Wf ∆𝐻 = 𝑚𝑐s ∆𝑇 Heat (Q) Q = ∫ 𝑇𝑑𝑆 (for any ideal gas or vapor as working substance) Q = mc∆T (for any ideal gas as working substance) Where: c = specific heat of gas (depending upon the process involved during the change of state) ∆T = change in absolute temperature 𝑄̇ is constant during a process: Q = 𝑄̇∆t Where: ∆t = process time interval = t2 – t1 𝑄̇ is not constant during a process: 82 𝑄 = w 𝑄̇𝑑𝑡 8x Mechanical Work (W) W = 𝐹𝑑 Mechanical Power (𝑊̇ ) y zP = 8 = 8 = F𝜐 Non-flow work (Wn) Thermodynamic properties vs. energy transfer mechanisms Example of point function: change in volume O w 𝑑𝑉 = 𝑉O − 𝑉V = ∆𝑉 O Wn = ∫V 𝑝𝑑𝑉 V Example of path function: work 𝛿𝑊 = 𝑊V|O ≠ ∆𝑊 Steady Flow Energy Equation Ein = Eout PE1 + KE1 + U1 + P1V1 + Q = PE2 + KE2 + U2 + P2V2 + Ws Notes: Heat(Q) • (+) if heat is added to the system by the surroundings • (–) if heat is rejected by the system to the surroundings Steady flow work (WS) • (+) if work is done by the system o Turbine • (–) if work is done to the system o Compressor o Pump Non-Flow Energy Equation Q = ∆𝑈 + 𝑊/ Notes: Heat(Q) • (+) if heat is added to the system by the surroundings • (–) if heat is rejected by the system to the surrounding Non flow work (Wn) • (+) if work is done by the system • (–) if work is done to the system Power Conversion 1 hp equals 33000 ft-lbf /min 550 ft-lbf /sec 42.4 BTU/min 2544 BTU/hr 0.746 kW