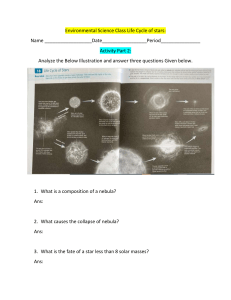
1 Fig 1.1 shows a speed – time graph of an object. The object was projected vertically upwards from the ground and held briefly at its maximum height. It was then released to fall to the ground. Ignore air resistance (g = 10m/s2 ) (a) Use the graph to determine the time (i) Taken by the object to reach its maximum height Ans: 1.5 seconds (ii) The object was held at maximum height Ans: 1.0 seconds (b) Calculate the maximum height reached by the object. 𝟏 h = gt2 𝟐 𝟏 h = (𝟏𝟎)(𝟏. 𝟓)𝟐 𝟐 Ans : h = 11.25m (c) The mass of the object is 0.5kg (i) Calculate the kinetic energy of the object as it leaves the ground k.e = 𝟏 𝟏 𝟐 𝒎𝒗𝟐 = (𝟎. 𝟓)(𝟏𝟓𝒎/𝒔)𝟐 𝟐 = 56. 25 joules (ii)State whether the maximum gravitational potential energy of the object is equal to, greater than or less than the maximum kinetic energy of the object. Explain your answer Statement : equal to Explanation : energy gained is equal to energy lost QUESTION 2 (a) Fig 2.1 shows a liquid- in – glass thermometer Name the parts labelled A and B A : bulb B : vacuum (b) Fig 2.2 shows a thermometer that used a bimetallic strip to measure the temperature of a domestic oven. The bimetallic strip is fixed at point P. a movable pointer is attached to the other end of the strip. (i)Which physical property varies with temperature in the thermometer in Fig 2.2? Ans : volume/ length of strip/ thermal expansion/ contraction (ii)Describe how the thermometer works when the oven is switched on Ans: The bimetallic strip is heated, and metal B will expand more than metal A. The pointer moves in a clockwise direction QUESTION 3 (a) Define the term amplitude Ans : The measure of the displacement of a wave from its rest position (b)Fig 3.1 shows a displacement – time graph for a sound wave Use Fig 3.1 to determine the period of the wave Ans: period = 𝟐.𝟓 𝟐 = 1.25s (c)The frequency of the wave is increased, and the loudness remains the same. On Fig 3.1, draw one complete wavelength to show the new sound wave. (d)A whistle produces a sound wave of frequency 256Hz. The speed of the sound in air is 340m/s. Calculate the wavelength of the sound wave 𝝀= 𝝀= 𝒗 𝒇 𝟑𝟒𝟎𝒎/𝒔 𝟐𝟓𝟔𝑯𝒛 𝝀 = 𝟏. 𝟑𝟑 QUESTION 4 Fig 4.1 shows a method of making a permanent magnet. A plotting compass is placed beside the magnet. (a)State two properties of permanent magnets 1. They have poles 2. They are ferromagnetic materials (b)(i)State the name of the method used in Fig 4.1 Ans: Electrical method (ii)Suggest the name of metal X Ans: iron/ steel (iii)Name another method which can be used to make a magnet Ans: Stroking method (c)Switch S is closed and the compass needle points as shown in Fig 4.1 State the name of the pole formed at end Q Ans: North pole QUESTION 5 Fig 5.1 shows an electric circuit Calculate (a) The total resistance of the circuit 8Ω + 4Ω = R1 Total resistance = 𝑹𝟏 ×𝑹𝟐 𝑹𝟏 +𝑹𝟐 R1 = 12Ω Rtotal = R2 = 6Ω Rtotal = 72𝛺 18𝛺 = 4Ω Ans = 4Ω b)the current in the 6Ω resistor current = 𝒗𝒐𝒍𝒕𝒂𝒈𝒆 𝒓𝒆𝒔𝒊𝒔𝒕𝒂𝒏𝒄𝒆 12𝛺 ×6𝛺 12𝛺+6𝛺 = 𝟔𝑽 𝟔𝜴 = 1A (c)the potential difference across the 8Ω resistor Current across 8Ω resistor is 0.5 A Why? Current across resistors in series is equal , so the current = 𝟔𝑽 𝟖𝜴+𝟒𝜴 0.5A P.D = current × resistance P.D = 0.5A × 8Ω Ans= 4V QUESTION 6 A hospital keeps some radioactive sources for medical purposes. (a)State one safety precaution when handling the sources Ans: always wear protective clothing. For example, lab coat (b)State one danger of being exposed to radioactive emissions Ans: They can cause cancer, gene mutation, Leukemia or even lead to sterilization (c)Suggest one way of safely disposing the sources from the hospital Ans * Store in lead *Concrete containers and bury underground *Lead shielding QUESTION 7 Choose one substance from the list to fit each given description. You may use each substance one, more than once or not at all. Sodium carbonate sodium hydroxide Copper(II) carbonate chlorine copper(II) sulphate ammonia (a)It is a solid that dissolves in water to form an alkaline solution = Ans: Sodium carbonate (b)It is a solid that is soluble in water and reacts with aqueous barium nitrate to form a white precipitate Ans: Copper(II) Sulphate (c)It is a gas that bleaches damp litmus paper Ans: Chlorine (d)It decomposes on heating to form carbon dioxide Ans: Copper(II) Carbonate (e)It is used to sterilize water Ans : chlorine QUESTION 8 Fluorine, Chlorine, Bromine and Iodine are elements in group VII of the periodic table. (a)Give a reason why these elements are placed in group VII of the periodic table Ans: They have 7 electrons on the valence/outermost shell (b)(i)Describe the trend of the reactivity of the elements in group VII Ans: They get less reactive as you go down the group (ii)Explain your answer in (b)(i) Ans: The halogens react by gaining an electron so if they have more shells it would be difficult to attract to the nucleus thus being less reactive c)Hydrochloric acid reacts with magnesium ribbon to form magnesium chloride and hydrogen gas . (i)write a balanced chemical equation for the reaction. Include the state symbols 2HCl(aq) + Mg(s) MgCl2 (aq) + H2(g) (ii)State two changes that can be made to increase the rate of reaction in (c)(i) • Warm the acid • Cut up magnesium ribbon into small pieces or alternatively use magnesium powder of the same mass • Use concentrated acid (iii)Hydrochloric acid is reacted with aluminium metal. Bubbles are produced slowly at the start of the reaction and then rapidly as the reaction progresses. Give an explanation for this observation Ans: The acid has to react with aluminium oxide layer formed on the surface of aluminum first. Once the acid has removed this layer, the reaction speeds up significantly QUESTION 9 Excess Magnesium Carbonate is added to 25.0cm3 of 2.00mol/ dm3 nitric acid. The equation for the reaction is: MgCO3(s) + 2HNO3(aq) Mg(NO3)2(aq) +CO2(g) +H2O(l) (a)Name the process used to remove the excess magnesium carbonate from the mixture Ans: Filtration (b)Calculate the number of moles in 25.0cm3 of 2.00mol/dm3 nitric acid Number of moles = concentration × volume c = 2.00mol/dm3 volume = 0.025dm3 number of moles = 2.00 × 0.025 = 0.05 moles c) use the equation and the answer in (b) to calculate the number of moles of magnesium nitrate formed 2 :1 0.05 : x 2𝑥 2 = 0.05 2 = x = 0.025 moles d) (i) The solution of magnesium nitrate from the reaction is evaporated and 6.9g of hydrated magnesium nitrate, Mg(NO3)2 . 6H2O is obtained The equation for the formation of hydrated magnesium nitrate is Mg(NO3)2 + 6H2O 1 Mg(NO3)2.6H2O : 1 0.025 : x x = 0.025 moles (ii)Calculate the mass of 1 mole of hydrated magnesium nitrate, Mg(NO3)2.6H2O Ans: The ratio is 1:1 , so one mole of hydrated Magnesium Nitrate is Mg = 24 H = 12×1 N = 14 × 2 O = 12 × 16 ( 24 + 28 + 192 + 12) = 256g Note : There is one Magnesium, 2 Nitrogen, 12 Oxygen and 12 Hydrogen atoms (iii)Use your answers to (d)(i) and (d)(ii) to calculate the mass of the hydrated magnesium nitrated formed. Ans: 0.025 × 256 = 6.4g (iv)Calculate the percentage yield of the hydrated magnesium nitrate 𝟔.𝟒 𝟔.𝟗 × 𝟏𝟎𝟎% = 𝟗𝟐. 𝟖% QUESTION 10 Ethene, C2H4 is an unsaturated compound. (a)State the meaning of unsaturated compound Ans: organic chemical compounds whose molecular structure contains one or more carbon – carbon double or triple bonds (b)Ethene reacts with bromine water and decolorizes it. Draw the structure of the compound formed from the reaction. (c)The equation for the addition of steam to ethene is: C2H4 + H2O C2H5OH Give any two conditions for the reaction 1.High temperature 2. Catalyst (d)Draw a ‘dot and cross’ diagram to show the structure of a molecule of carbon dioxide QUESTION 11 Fig 11.1 shows a cross section of the human heart (a)State the name of the structures labelled V and X V : Right Atrium X : Aorta (b)Explain how the structure of the left ventricle enables it to pump blood to distant parts of the body. Ans: it is muscular. It is also bigger in size, so it is able to generate more pressure. c)State two structural differences, apart from presence of valves, between the parts labelled W and Y. 1. Structure W has thick walls while Structure X has thin walls 2. Structure W carries oxygenated blood while structure X carries deoxygenated blood Note: W – artery X – vein (c) On Fig 11.1 use arrows to show the movement of deoxygenated blood in and out of the heart. QUESTION 12 Beer contains a drug that is commonly abused (a)Define the term drug Ans : A medicine or other substance which has a physiological effect when ingested or otherwise introduced into the body. b)Name the drug contained in beer Ans: alcohol c)Fig 12.1 shows the hands of two persons, L and M, performing an experiment to demonstrate the reaction time of M before drinking beer. L releases the metre rule and M catches it at point A. State and explain what is observed if the experiment is repeated 30 minutes after M drank a lot of beer. Observation: the ruler will be caught at a position higher than A Explanation : alcohol suppresses nerve cells in the brain causing them to slow down and this leads to increased reaction time QUESTION 13 Fig 13.1 shows the human digestive system A boy eats solid food that contains carbohydrates, proteins and fats. (a)State the name of the process through which food is taken into P Ans: ingestion (b)State the name of the process which makes food move through Q Ans: peristalsis (c)Fig 13.2 shows bar charts representing proportions of nutrients in a food sample obtained at the end of digestion in each region of the alimentary canal. Letters W, X and Y represent regions of the alimentary canal (i)Which section of the bar chart is for the sample obtained from the duodenum? Ans: W (ii)Explain your answer in (c)(i) Ans : Sugars and peptides are available in large amounts as compared to proteins and starch which shows that protease has acted on proteins and pancreatic amylase on starch . QUESTION 14 Fig 14.1 shows a nerve cell found in an organism (a)Define the term organism Ans: a group of organs and systems working together to make an individual plant/ animal. (b)State the name of the nerve cell in Fig 14.1 Ans: Sensory neurone (c)Identify the parts labelled A and B A : Cell body B : Nerve fibre (d)The normal concentration of glucose in human blood is 100mg/dm3 . Suggest how a decrease in the concentration of glucose may affect the activity of the cell in Fig 14.1 Ans: Glucose is required for energizing body cells therefore a decrease in its concentration would mean that the cell would not be able to effectively carry impulses from the sense organs to the Central Nervous System QUESTION 15 Fig 15.1 is a graph showing water loss by a plant on two different days (a)State the name of the part of the leaf through which plants lose water Ans: Stomata (b)Describe and explain the shape of the graph for day 1 Ans: Water loss increases from midnight to midday and is at its peak at midday because temperatures are high, and stomata is open to allow diffusion of carbon dioxide for photosynthesis. The water loss then decreases from midday to midnight because temperatures drop, and stomata closes due to reduced light intensity (c)Suggest one environmental condition which could have resulted in the graph obtained in day 2. Explain how the condition affected the rate of water loss Environmental condition: Humidity Explanation : There is more moisture in the atmosphere and as such less water is lost from leaves due to moisture concentration on the atmosphere