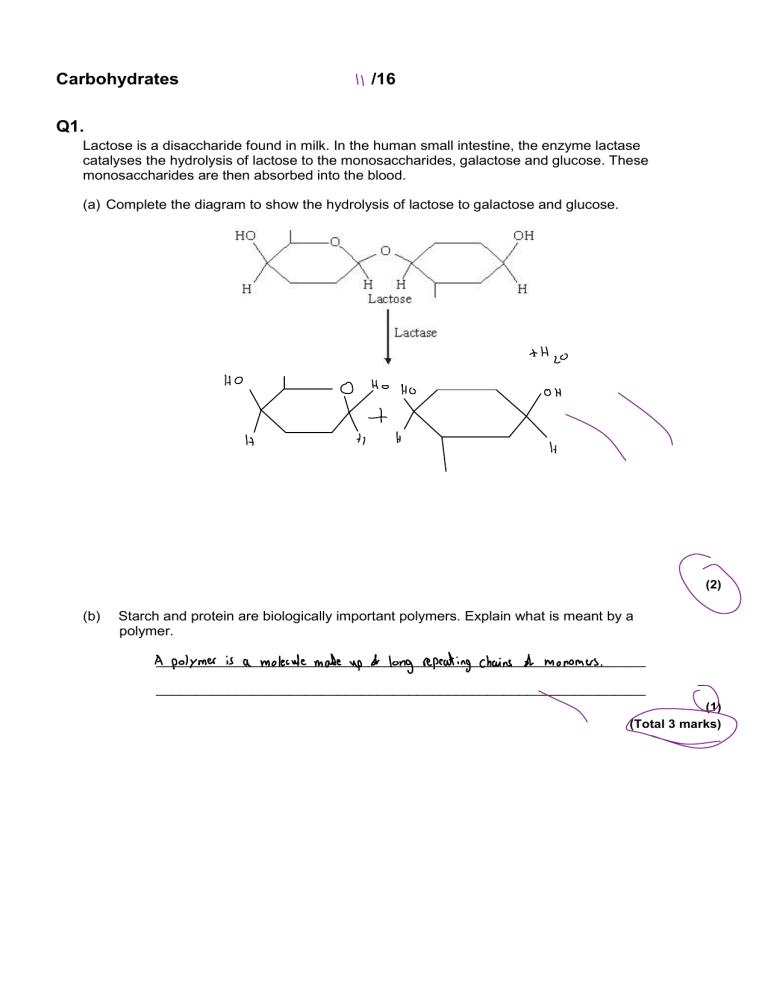
Carbohydrates " /16 Q1. Lactose is a disaccharide found in milk. In the human small intestine, the enzyme lactase catalyses the hydrolysis of lactose to the monosaccharides, galactose and glucose. These monosaccharides are then absorbed into the blood. (a) Complete the diagram to show the hydrolysis of lactose to galactose and glucose. * Ho O 1 H Ho H+ Ho H Heo OH 1, 0 (2) (b) Starch and protein are biologically important polymers. Explain what is meant by a polymer. long repeating A ______________________________________________________________ chains at monomers polymer is a molecule made up of . ______________________________________________________________ \ 0 (1) • (Total 3 marks) Q2. Scientists investigated the hydrolysis of cellulose from samples taken from recycled paper. They measured the concentration of the products formed in the reaction. (a) Name the monomer that forms a cellulose molecule. \ b___________________________________________________________________ glucose (b) (1) 0 Describe a biochemical test for the monomer you named in your answer to question (a). test ___________________________________________________________________ ☐ addicts reagent : \ Add 2cm A food sample 1)___________________________________________________________________ ] Add equal amount of Bwdicti ___________________________________________________________________ 2) reagent \ ___________________________________________________________________ 3) place in hot water bath for 5 minutes ' . ___________________________________________________________________ 7) If color becomes : green yellow, orange or rod then it is found , . , ___________________________________________________________________ 0 (2) (c) In the investigation, the scientists mix the enzyme cellulase with the paper and a buffer solution in an incubated flask. I To measure the progress of the hydrolysis reaction, samples are taken from the flask every twelve hours. The concentration of the products in each sample is measured. The table shows the scientists’ results. 62.5 - 61.8 (72×60)-(-6-1160) =a? (d) Hours Concentration of products / g dm 3 12 24 36 48 60 72 28.2 42.9 55.7 59.2 61.8 62.5 Rate of hydrolysis of cellulose / g dm 3 minutes 1 39.2 × 10–3 20.4 × 10–3 17.8 × 10–3 4.9 × 10–3 3.6 × 10–3 Calculate the missing rate of hydrolysis. Write your answer in the table. Plot a suitable graph of the rates of hydrolysis as shown in the table. I(1) To ✗ E- ¥-0 ' ✗ § ✗ ɧ¥§É¥ ' ¥ ¥ ✗ \ ✗ E. 12 (e) 24 48 36 ① 72 60 Hours (3) Describe and explain the results of this investigation. Tk ___________________________________________________________________ more time that passes the higher the concentration of products this , ___________________________________________________________________ molecules into smaller products Another is becase d hydrolysis turning larger . ___________________________________________________________________ the time tie lower the rated hydrolysis is This is thing is that the longer . be ___________________________________________________________________ case the amount A large molecules that can be hydrolink is decreasing , ___________________________________________________________________ meaning four things can be hydro libel steep decrease and then a less rapid • . ___________________________________________________________________ decrease amount A High initial rate d reaction at 12 hours as a large . cellulose Decreased rate A Nation be case more cellulose was hydrolysed few cellulose left ___________________________________________________________________ . y , (3) (f) Explain how the structure of cellulose is related to its role in plant cell walls. ' cellulose is used to keep the plants structure ___________________________________________________________________ rigid it forms mic roti birds , strong that ___________________________________________________________________ are connected with strong hydrogen bonds These micrdi birds are very . and ___________________________________________________________________ insoluble strong molecules to hold the structure making great • Attendant Forms long wnbrancek chains CN B- glucose) ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ 2 ___________________________________________________________________ , (3) (Total 13 marks) I