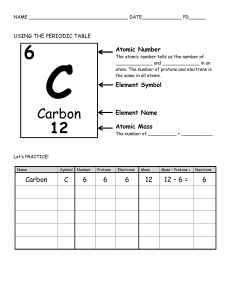
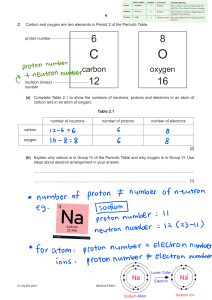
Identifying the parts of an Atom PhET Lab Introduction: Atoms are the smallest things that retain the properties of matter we can observe. Atoms are made of three subatomic particles; protons, neutrons, and electrons. • Protons have a mass of ___________ unit and a charge of ___________. • Electrons have a mass of nearly___________ unit and a charge of ___________. • Neutrons have a mass of ___________ unit and a charge of ___________. (we will talk about them Later) In this simulation, you will build atoms, subatomic particle by subatomic particle and observe the effect of adding more of each particle. Procedure: Begin by playing with the simulation for a while. Become familiar with the interface. To start over, click Show the symbol, atomic mass, and charge by clicking on the . 1. Click on the + sign for each of the boxes (element name, net charge and mass number) to view changes as you change the number of particles in the atom. 2. What happens when you add protons, neutrons, or electrons? 3. What particle(s) are found in the center of the atom? ______________________________________________________ 4. Play until you discover which particle(s) determine(s) the name of the element you build. _________________________ 5. What is the name of the following atoms? a. An atom with 3 protons and 4 neutrons: _______________________________________________ b. An atom with 2 protons and 4 neutrons: _______________________________________________ c. An atom with 4 protons and 4 neutrons: _______________________________________________ 6. Fill in the blanks below to show your results: a. Neutral atoms have _______________________________________________protons and electrons. PART II: SYMBOL SCREEN ( GO BACK TO THE HOME SCREEN) 1. Using the Symbol readout box, figure out which particles affect each component of the atomic symbol and how the value of the numbers is determined. d b a Position in symbol box Term to describe this information Particle used to determine this How the value is determined a Element symbol protons # of p will identify the element b charge c Atomic number d Mass number Protons plus electrons Protons, neutrons Draw it out! Draw the symbol boxes as shown on the simulation below. Hydrogen: H Carbon: C Oxygen: O Neon: Ne Atomic number- 1 Atomic number- 6 Atomic number- 8 Atomic number- 10 Mass number- 1 Mass number- 12 Mass number- 16 Mass number- 20 Analysis Questions 1. Adding or removing protons from an atom does what to the atom? ____________________________________ 2. An atom with the same number of protons and electrons has a charge of _______________________________. 3. Adding two electrons to a neutral atom produces with a charge of _______________________________. 4. An atom with six protons and five electrons would have a charge of _______________________________. 5. What atom is created with nine protons, nine neutrons, and nine electrons? _______________________________ 6. Show the full symbol for the above atom in question 5 in the box below. The Game Complete the table below Protons Neutrons Electrons 4 4 5 Atomic Number Mass Number Element name 4 8 Berylliu m 8 3. 11 4 5 6 1. 2. Full Symbol (Including the charge) Be 4 B 5 8 8 7 4. 5. 6. 15 O 8 7. 8. 9. 7 13 10. 13 N 7 11. 12. 13. 9 20 14. 20 F 9