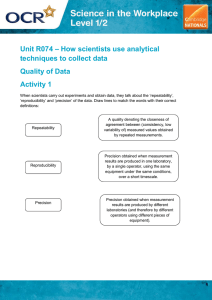
This Is Why There Are So Many Ties In Swimming For each paragraph write one sentence that summarizes the paragraph or a question it poses. Olympics 2016, three legends of swimming—Michael Phelps, Chad Le Clos, and László Cseh—turned in identical times to share silver in the 100m butterfly. Last night, Simone Manuel tied for gold with Canadian Penny Oleksiak in the 100m freestyle. Modern timing systems are capable of measuring down to the millionth of a second—so why doesn’t FINA, the world swimming governing body, increase its timing precision by adding thousandths-ofseconds? As it turns out, FINA used to. In 1972, Sweden’s Gunnar Larsson beat American Tim McKee in the 400m individual medley by 0.002 seconds. That finish led the governing body to eliminate timing by a significant digit. But why? In a 50 meter Olympic pool, at the current men’s world record 50m pace, a thousandth-of-a-second constitutes 2.39 millimeters of travel. FINA pool dimension regulations allow a tolerance of 3 centimeters in each lane, more than ten times that amount. Could you time swimmers to a thousandth-of-asecond? Sure, but you couldn’t guarantee the winning swimmer didn’t have a thousandth-of-asecond-shorter course to swim. (Attempting to construct a concrete pool to any tighter a tolerance is nearly impossible; the effective length of a pool can change depending on the ambient temperature, the water temperature, and even whether or not there are people in the pool itself.) Sports that subject athletes to an identical course— bobsled, for example—can use thousandths because this question doesn’t matter. Speed skating uses thousandths, though given how start commands are issued in that sport and the incredibly slow speed of sound, maybe they shouldn’t. Summarize the article in one paragraph: Explain the role of error and precision in measuring times at the Olympics: Accuracy & Precision Worksheet Accuracy: refers to how close a measurement is to a true, accepted or target value. Precision: Refers to the reproducibility of a series of measurements 1. The following measurements were made to determine the density of a material whose value was, according to the Handbook of Chemistry and Physics, 1.24 g/mL Trial #1 Trial #2 Trial #3 1.20 g/mL 1.22 g/mL 1.22 g/mL a. make a general comment on the accuracy of these results b. make a general comment on the precision of these results c. what may have caused these results? 2. The following measurements were made to determine the density of a material whose value was, according to the handbook of Chemistry and Physics, 1.15 g/mL Trial #1 Trial #2 Trial #3 0.95 g/mL 1.16 g/mL 1.26 g/mL a. make a general comment on the accuracy of these results b. make a general comment on the precision of these results c. what may have caused these results? 2. The following measurements were made to determine the density of a material whose value was, according to the handbook of Chemistry and Physics, 3.75 g/mL Trial #1 Trial #2 Trial #3 4.75 g/mL 4.76 g/mL 4.74 g/mL a. make a general comment on the accuracy of these results b. make a general comment on the precision of these results c. what may have caused these results? Place 4 dots on each target with the appropriate level of accuracy and precision. On Your Own! a. Create one image representing accuracy but not precision b. Create a second image representing precision but not accuracy c. Create a third image representing accuracy and precision. Cannot use a target or bulls-eye!!