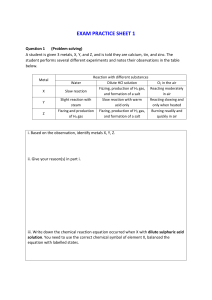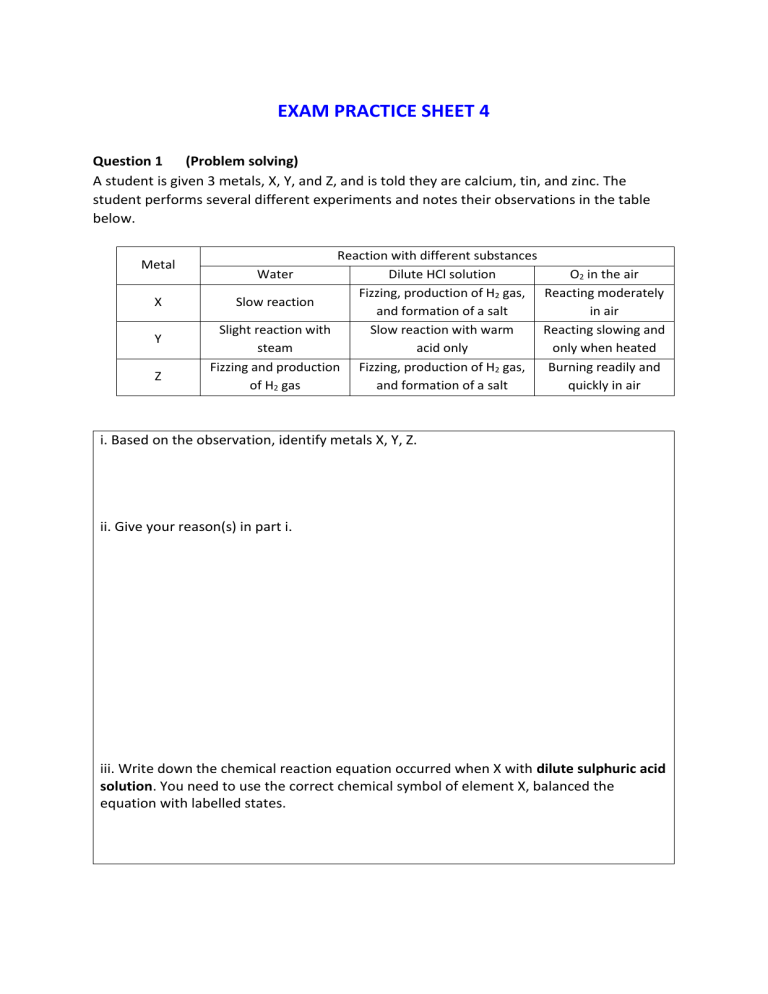
EXAM PRACTICE SHEET 4 Question 1 (Problem solving) A student is given 3 metals, X, Y, and Z, and is told they are calcium, tin, and zinc. The student performs several different experiments and notes their observations in the table below. Metal X Y Z Reaction with different substances Water Dilute HCl solution O2 in the air Fizzing, production of H2 gas, Reacting moderately Slow reaction and formation of a salt in air Slight reaction with Slow reaction with warm Reacting slowing and steam acid only only when heated Fizzing and production Fizzing, production of H2 gas, Burning readily and of H2 gas and formation of a salt quickly in air i. Based on the observation, identify metals X, Y, Z. ii. Give your reason(s) in part i. iii. Write down the chemical reaction equation occurred when X with dilute sulphuric acid solution. You need to use the correct chemical symbol of element X, balanced the equation with labelled states.