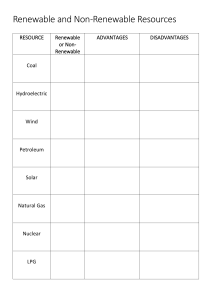
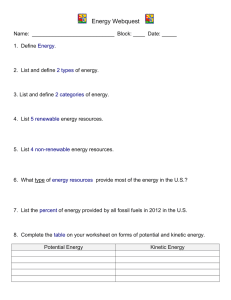
Name: Total /25 Environmental Science Unit 3: Energy Quiz PART A: MULTIPLE CHOICE. Circle the response that is correct or best answers the question. The following information relates to Qs 1 and 2: The process that occurs in a power station and the efficiency of each conversion is shown below. natural gas → boiler → steam → turbine → rotation → generator → electricity 60% 80% 85% Question 1 The overall efficiency of the plant will be approximately A. 25% B. 40% C. 75% D. 225% Question 2 The law of conservation of energy means that the plant will A. convert all the energy in the natural gas to electricity. B. be highly efficient in converting one form of energy into another. C. convert all the energy in the natural gas into other forms of energy. D. be sustainable, since it will conserve unneeded energy for future use. Question 3 The term .exothermic. refers to a reaction A. in which heat is given out. B. in which heat is absorbed. C. which proceeds very rapidly. D. which requires high temperatures before it begins. Question 4 Which of the following groups contains only examples of renewable energy sources? A. hydroelectric, coal, wind B. nuclear, natural gas, solar C. solar, wind, hydroelectric D. hydroelectric, natural gas, wind Question 5 Natural gas is an example of A. a fossil fuel, renewable energy source. B. a non-fossil fuel, renewable energy source. C. a fossil fuel, non-renewable energy source. D. a non-fossil fuel, non-renewable energy source. Question 6 The burning of natural gas is an example of A. an exothermic reaction. B. an endothermic reaction. C. conversion of heat energy to kinetic energy. D. conversion of mechanical energy to electrical energy. Question 7 The water stored in Lake Jindabyne, one of the dams in the Snowy Mountains Scheme, to generate hydroelectric power is an example of A. kinetic energy. B. potential energy. C. mechanical energy. D. non-renewable energy. Question 8 Within a hydrogen fuel cell, each kilogram of hydrogen gas used (with excess oxygen) contains approximately 13 000 kJ of chemical energy, and in the fuel cell produces 3 500 kJ of electrical energy. The percentage efficiency of conversion of the fuel cell is A. 0.27% B. 7.3% C. 27% D. 73% Question 9 Power generated by a wind turbine is an example of the conversion of: A. kinetic energy to mechanical energy to electrical energy B. potential energy to mechanical energy to electrical energy C. kinetic energy to mechanical energy to chemical energy D. potential energy to kinetic energy to electrical energy The following information relates to Qs 10-12 An engineer is evaluating energy source options for a city. She recommends that the following options would be feasible. Option 1 . A nuclear (uranium) powered generating plant Option 2 . Wind power generating plant Option 3 . Natural gas powered generating plant Question 10 Which one of the following is an advantage of the nuclear plant (Option 1) compared with the natural gas plant (Option 3)? A. Uranium is a renewable energy source. B. Uranium is in plentiful supply in most countries. C. A nuclear plant is more acceptable in a populated area. D. No carbon dioxide is produced during operation of a nuclear plant. Question 11 Which one of the following is an advantage of natural gas (Option 3)? A. It is renewable. B. It is non-renewable. C. There are many sources worldwide. D. It produces no carbon dioxide greenhouse gas emissions when burned. Question 12 Which one of the following is a disadvantage of wind power (Option 2)? A. It is renewable. B. It is non-renewable. C. It produces no greenhouse gas emissions. D. It will only produce electricity part of the time. PART B: SHORT ANSWER. Write your answer in the space provided. Show all workings and units. Question 1 A student makes the following statement: .Fossil fuels are non-renewable, while non-fossil fuels are all renewable. Explain why this statement is incorrect. Give one example to support this. 2 marks Question 2 The two main types of energy are kinetic energy and potential energy. Explain the difference between these two types of energy. 2 marks Question 3 Calculate the amount of power used by a small bar heater using 1000 J/sec. Show your workings and include units. 2 marks Question 4 Explain the implications the first two Laws of Thermodynamics have on the quantity and quality of energy during energy conversions/transformations. 4 marks Question 5 Calculate the efficiency. For every 100J of energy put in; 35J of work is done by a diesel engine 1 mark Question 6 The energy conversion of natural gas to heat is about 75%. One cubic metre of natural gas stores 39 MJ of chemical potential energy. State the amount of energy converted to heat from natural gas in a domestic wall furnace. 2 marks