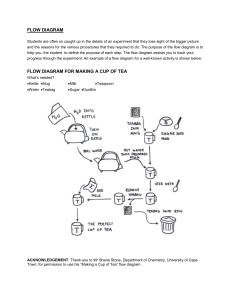
Flying Teabags! Objective Students will learn how convection will make a teabag fly Unused Teabag one with a string and staple Matches Piece of aluminum foil or a glass plate Sharpie Scissors Googles I strongly recommend that you perform this experiment outside on a windless day. Usually the teabag does not fly higher than the ceiling, but it has happened. 1. Remove the staple and string from the teabag. Be careful not to rip the bag 2. Use scissors to cut the top of the teabag. Empty the contents of the bag. Unfold and straighten the empty teabag. You should have a long cylinder. Try to make it perfectly round because it will fly higher. Draw a snowman on the cylinder. Put on safety equipment. 3. Place the snowman on the foil. Light the top of the bag all the way around the edge. As it is burning ask the students what they think the heat is doing to the air molecules both inside and outside the column. 4. The bag will go into the air quickly and come back to earth. It should rise rapidly and descend slowly as it cools. I always encourage students to video this lab. 5. Let the burn completely burn to ash. Before students begin their written assessment, I would recommend having them draw a diagram of the gas molecules on the board. Also any videos they have taken will be a great help to them to review this lab. Directions https://youtu.be/bixDlU8edGw Name Define the following terms A. Kinetic Energy B. Thermal Energy Flying Teabags Why did it fly? List three reasons. ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ ___________________________________________________________________ __________________________________________________________________ Answer Key 1. Kinetic Energy which a body possesses by virtue of being in motion. 2. Thermal Energy is the energy that is generated and measured by heat. An example of thermal energy is the kinetic energy of an atom. An example of thermal energy is the chemical energy in a molecule. ... (engineering) A form of energy; sensible energy; heat. #1 There are three reasons why it flew. There was a density difference between the air inside the cylinder and the air outside. When the flame went down the cylinder the air was trapped in the bag. The molecules began to move extremely fast and to spread out both inside and above the cylinder. The molecules inside were much farther apart than the molecules outside the cylinder. Warmer less dense air rises above cooler denser air. #2 The burning teabag produces hot less dense air inside and above it. This creates a convection current of rapidly rising hot air above the flames. The larger volume of space generated by the hot rising air inside the cylinder needed to be filled. The cooler denser air outside the burning teabag moves in from the bottom to fill the space under the heated air #3 After the bag has burned it becomes both smoke and ash. The hot smoke rises lifts away and dissipates into the air. There may be a delicate ash frame of the bag remaining but because the ash frame is so lightweight the force of rising hot air is strong enough to quickly life it upward. As it rises it cools and if you are lucky will fall back on the aluminum foil Teacher notes When I first began to teach, we would use Ditto paper. It was printed purple ink and freshly printed copies produced an unforgettable smell. Years later I discovered that both the ink and Ditto machine solves were highly toxic to humans. Back in the 1960 ‘s no one was at all worried about Ditto paper.