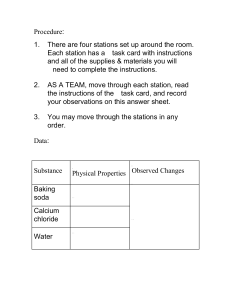
Baking Soda Vinegar Lab Group A Purpose/ Statement/Question: Hypothesis: Identification of Variables: ● Independent Variable: ● Dependent Variable: ● Constants: Experiment: A.Materials: B. Step: Record Data: Mass of Baking Soda Trial 1(time in seconds) Qualitative data: Few Questions to Analyse: Trial 2(time in seconds) Trial 3(time in seconds) What happens when vinegar is added to baking soda? What are the products of vinegar and baking soda? How will your result change if you add soda to water? What are the side effects/benefits of drinking baking soda and water? Which combination seems better for baking a cake? Conclusion: To write a good conclusion you must: state what your results show (and describe the shape of the graph if you have one); Use numbers to describe the trend/pattern, compare your results to your hypothesis; explain your results with scientific knowledge. Evaluation: Answer and explain these questions: Did you carry out the experiment accurately and how you know? Did you make any errors and how? Did you control the controlled variables well? How could you improve the method so that your experiment was more accurate? reference list: Lab Report Submitted by: Rubrics 1-2 3-4 5-6 7-8 You can correctly You can correctly You can correctly Collects and You can collect, collect, organize, collect, organize, Presents Data and present data collect, and and present data transform, and (cannot be achieved present data (cannot on this task) be achieved on this task) You can accurately You can accurately You can accurately You can interpret data interpret data interpret data interpret data and outline results and outline results and outline results but did not use evidence in your using evidence in using evidence in using evidence in conclusion. your conclusion. your conclusion your conclusion and scientific and correct scientific reasoning. (cannot reasoning. (cannot be achieved on this be achieved on this task) task) but have some obvious errors. Interprets Data present data Science behind it Baking soda often called by other names such as sodium bicarbonate or bicarbonate of soda. (NaHCO3) Baking soda is an alkaline substance when mixed with acids change the pH levels and that is the reason it became a quickest remedy for illnesses like stomach upset, bad smell etc. The process of combining baking soda and vinegar forms a cloudy liquid and produces carbon dioxide The bubbles and foam you see are filled with carbon dioxide gas (CO2) that’s being released by an acid/base reaction. Vinegar is acetic acid dissolved in water and baking soda is a base called sodium bicarbonate. Initially, the reaction makes carbonic acid which is unstable. It quickly breaks down into CO2 and water. The gas then rapidly leaves the water creating foam and bubbles along the way. If you want to learn more check these sites: https://handsonaswegrow.com/baking-soda-vinegar/ https://gosciencegirls.com/baking-soda-vinegar-reaction-with-heat/