-
I (
1 · T t1e ce ll
Table 1.2
• Wllich structures are found In plant cells only?
• Give four differences between plant cells and
animal cells.
Diffe rences between plant and animal cells.
P lan t cell
has a rigid ce ll wa ll so little variation in
ce l l shape
usua ll y has chloroplasts
usually has large centra l vacuole, and
may also ha ve other sma ll vacuoles
food stored is stard1
Which structures are common to both plant
cells and animal cells?
A nima l cell
has no cell wa ll so more .
.
vanatio
shape accord111g to funct ion
n Of cen
has no ch loroplasts
food stored as glycogen
Practical activity 1.1
Observing typical cells
To get a good mark for your drawing, make
sure of the following.
• It has a title.
• It is a large drawing with continuous lines.
Don't sketch the lines. Use a pencil with a
sharp point!
• Label lines are drawn to one side and
end at the same distance from the side of
drawing. Use a ruler to draw them!
• Labels are written with a pencil either in
all capital or all common letters.
• The magnification is written usually to the
bottom right of the diagram.
Observing an animal cell
I
Use a clean fudge stick or wooden spatula to gently scrape the
inside of your cheek.
2 Place the scrapings on a microscope slide and add two drops of the
stain methylene blue.
3 Cover the sample with a cover slip and observe under a
microscope, first using a low power to find the cell sample and
then under a high power to observe an indiv idual cheek cell.
4 Draw the cheek cell and label it.
Observing a plant c ell
I
2
Slice an onion into two le ngthw ise a n d
Use tweezers or forceps to pull a way
the inner surface of the leaf.
;-c,-, ;,H 'e
1h~' , 1,in
a n inner fleshy leaf.
li n ing/sheath from
3 Cut a very tiny strip, about 4mm sq uarE, (rum the sheath and
place it on a microscope slide .
4 Add a few drops of dilute iodine to s rn in the cells and cover the
sample with a cover slip.
5 Examjne the sample under low powe r and then under a high
power objective.
6 Draw and fully label one onion cell.
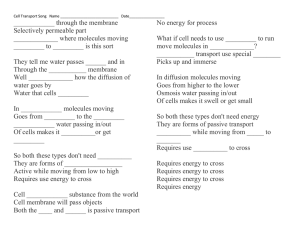
Transporting substances in and out of cells
II to another.
Substances are constantly moving in and out of cells or from one ce
are
This action is very important in order to keep cells alive. For example, r1ie;:uod
reactions taking place inside cells which require many substances that ar\ first
in the environment around the cell. These substances must enter the ce:d ;iring
passing through the cell membrane. Also, waste products are often pracluc
whl'
these reactions and must be removed from the cells. Can you think of a reason
it would be important to remove the waste products from cells?
1iarn1
Did your an·s wer include 'waste products are often toxic and may cause ck'
to or kill the cell if allowed to accumulate? ' If yes, you are on the right tra ·
4
Th e c e ll · 1
partiaUy permeable membrane
Th e ce ll membrane controls wh ich particles e nt e r or leave the ce lls. Pur thi s
reason it is described as a pa rtially permeable me mbra ne. The pa rticl es e nt e rin g
o r leav in g the cell may do so eith er by the process of diffu sion or by os mos is.
What is diffusion?
diffusion
Diffusion involves the movement of particles from a region of high concentra tion
(i.e . where th ere are many particles) to a region of lower concentration (i .e. where
there are fewer or no particles) until the particles are evenly distributed. Diffusion
happens in gases and liquids (see figures 1.4a and b).
(a)
gas jar filled with
bromine vapour and
covered with lid
the mouth of an empty gas jar
is greased and the jar inserted
over the first gas jar
(a) Diffu sion in a gas
can be shown using a coloured
gas. (b) Diffusion in a liquid can
be shown by placing crystal s of
dye at the bottom of a beaker o[
wa ter. Over tim e the molecul es
o[ coloured gas, or dissolved dye,
diffuse so they are even ly spread
throughout the volume available.
the lid is removed (from between
the two jars) and the bromine vapour
begins to diffuse into the top Jar
after a few hours the bromine gas
is evenly distributed between the two jars
Figure 1.4
-
,
I
I
I
\___
----
Figure 1.5 'The diffusion ex perience!'
5
l
1 · Tl1 e cell
Practical activity 1.2
Observing diffusion in a liquid
Half-fill a beaker with tap water.
With the aid of a dropper, carefully add two drops of dye (for
2 exa mple food colouring, ink, potassium permanganate solution
screened methyl orange) to the bottom of the water in the beaker
3 Observe the movement of the colour in the water and record all ·
your observations.
Questions
What was the colour of the dye used?
2 What was the colour of the water before the dye was added?
3 How did the dye move in the water?
4 What was the colour of th e water after one hour?
5 At the start, where in the beaker were the particles of the dye in
highest concentration? How were you able to tell where the poini
of highest concentration was?
6 Explain why the dye particles were able to move through the
water without being stirred.
7 Explain the distribution of the dye in the wa ter after one hour.
Diffusion across a cell membrane
concentration gradient
• Explain the term concentration gradient.
The diagram (figure 1.6) shows a box divided
by a partition with a small hole in it. One side
of the box contains many gas molecules of
one kind. The other side contains only a few of
these molecules. Explain what will happen to
the molecules and why.
6
Particles diffuse across a cell membril ne heca u, e the concentration of the diff
particles is greater on one side of the cel l mernb1 an<: tha n on the other. This dilfere
in concentration is known as a concen ff<1 iio11 gr,1die nt. As long as a concemra ·
gradienl is present diffusion will take place. Th.-·re lorc. as the particles become er
distributed across the membrJ1 1e diffu sinn s!t) WS do,vn or stops.
.J
Figure 1.6
EYamples of diffusion in living organisms
JJiff11,1i,m alth r11an y pF>tc~"\ i fl at 1JU;11 r III li 11lo~ ,,ry_,aui~,,,,,,
Gas excha nges In the a lveoli (ai r ,ac~, In t he lun~
(J xyg,,.:TJ d iff u~, lrtJm the hm~ IHJ.Jff/ 1,arri<h·•,1 1,, rJ,•· 1,k~·,tJ, ·11fwr,· 1ti,·w a n ·
''½ YgA; rl panidcl'J ''"'''' hg.un• l,7 J. rArl•m di1,-;,;1d,· tJiffu·..,-. 1,,,,,, 1lw 1,k,,,.d l11tany
carbtm rJi,,::.ddc J1ani,t<..._, u, tlw hut~ wtic:rc th,·n· are f,,w,·r, ,;,1,,.,r, rJ11,nrl, .. wu1f,i,•j,
r.J/J'/'~ ttorn !l'.1:
,~,J li':11,
;/,.//J~';;y'/
'A,{';// rJ,,,,/ ~, ~~11
l'?'"A ~"'
•111;rJ•?A"Alf,..~.:ir1 rJi!' ,-x;g
'"'"J"U;f,JIJ';t'Jl"r.f', t;,i>rl"_;
f',:,s1'// ,j,_,/ ij;,;A fj
'li';/"'1' l'//0;$'~-~//
, .,,,_;'Y.//;
'.J"//,,•;,1.,,
..Y,1~;,. 1:,,l,,-,,, '''l' '"11;.
t¼1r/••I':,, •//)/
,p,,.~..
;;r.,.~te,;! 1;1,,.,<1 u, the ,mygtn dcfidc'Jlt cdls in the
,,
•• 'ii
Qr/A - I ,,_, <'<rA
fff, ~r/'¥J(I
'/A1".H#;, -
..,/rl;t' ~.M .,(,
'iD>----- - - ~
-:..rrq,~Q?.//
arrl .. '/fr#'. .. '?~.r
€- 01'
lftt!?lr~,..ptfa:FA'ltJ/,.,A~ · h~a~/'1.iYA
ll'!illleP,"""-'llll~foOViMe f:;;Y,i,c •lllt'J
e;r!)rA clio/~ ditf.- f<Olll It>! !Y.J!ly'J o,IIJ
i<S!Jlll'i!ll<;,A
Figure / .B Uitru~,on of <JXYSffl from thee bl<.,c,d inw 1hc cdls.
7
~rm
1 Tlie cell
Photosynthesis is the process by which plants
make food from carbon dioxide (COJ and water
(H,O) in the presence of sunlight (see page t 6).
Gas exchanges in 11lants
Gus cxch,1ngL':-. in plant s Jbu () ( ( llf by lhe process of diffu ~ion . Carbon diu .
nccch:d by planb l o make food enters throu gh the stomatJ ol the lcaJ, Jn(! X1t1r
whic h is produced durin g photosynthesis exit s th e leaf via the sume roui/1XYKt~
' D<l"1Jy ~
co,
gas enters and exits the leaf
through the stomata! pore
{opening) of the leaf
Figure 1.9
Gas exchangc:s occur by diffusion in plants.
What is osmosis?
The cell membrane functions as a partially
permeable membrane.
Osmosis is a special case of diffusion. It is the diffusion of water moleculesacr°"
a parti_a lly permeab_le membrane such as a cell membrane. Water molecules
move m both d1recuons across the membrane, but th ere will be a net movemem111
~
wa~er molecul es from a region '"1 here there are many of them (dilute solution)ioa
region where there_ are fe w (concentrated solution) or no water molecules. Figu,
1.10 explains the differe nce between a dtlute solution and a concentrated soluti<XL
a diiute solution cl ~,
X t1as fev-.·er so'ute
molecules comparer.:
to the riumtc; cf
mo!er_,.;I··
Q0
0
QO
0
0 () O
Figure 1. 10
solution .
Aconcentrated solution has a greater
percentage of solute molecules dissolved in
the solvent.
A dilute has a smaller percentage of solute
molecules dissolved in the solvent.
8
T!,L •Jitk :-e ncc be1wee n a
a concentratoo
solution of Xhas
agreater rrnteof solute rrdeo.Jes
comparedto tte
numberof wat1:t
molecules
soluteX md,erulg
dil me solution and a concentrated
If yo u had a container that was di vided by a partially permeable membra~
and you put pu re wa te r o n one side and a concentrated sucrose (suga r) soluuoo
on the oth er, th en osmosis would occur (figure 1.11). Sugar molecules cann~
pass through the membrane because they are too large but water moleculesa~
smaller and can diffuse through.
Tl1e ce ll · 1
-
• What is osmosis?
• Which particles move across /he partially
permeable membrane during osmosis?
A dehydrated (withered) carrot was placed in
a bowl of cold water tor a few hours. After that
time the carrot looks fresh again and full of
water. Explain what happened by answering the
following questions.
(a) What process caused this change in the
carrot?
(b) Which molecules caused the change in the
carrot?
(c) What was the function of the cell
membranes in the carrot cells?
(d) At the start, which area had the higher
concentration of the molecules that caused
the change?
(e) At the start, which area had the lower
concentration of the molecules that caused
the change?
In which direction was the net movement of
these molecules?
net movement
of water
molecules
sugar water
f
pure water
~o oI..o~~
0
\...4
o
0~ , ~ () ()
o~ o o
partially permeable membrane
Figure 1.11
Key
water molecule
sugar molecule
0
Q
Note that the water will move
in both directions across the
partially permeable membrane
during osmosis. However the net
movement wi ll be towards the section
with fewer water molecules.
Osmosis is a special type of diffusion.
The pure water has more water molecules than the side with the sugar
solution; this sets up a concentration gradient. Even though the partia ll y
permeable membrane will allow water to move across it in both directions,
the net movement of water will occur from the side where there are more
water molecules (pure water) to th e side where there are fewer water molecules
(sugar solution) as illustrated in figure I. 11.
m
I
Practical activity 1.3
Observing osmosis
r:==::
I
II
n
I
1?
I
I
\...
£
/
Steps 1-2
I
Steps 3-4
Figure J,12 Steps showing
apparatus set-up for studying
osmosis.
I
I Cut a length of 1 ;~i;i: 1g t;:hi 1 1g Son lo ng and tie one end with a
piece of thread to uea1e ,7 hag.
the bag with a solmion of sodium chloride containing 30g of
sodium chloride to I00cm 3 of wa re r (fig ure 1.12).
3 Use another piece of thread to tie the bag to the bottom of a
capillary tube.
4 Clamp the capillary tube to a retort stand and gently lower the bag
of salt solution into a beaker of water as shown in figure 1.12.
5 Mark the level of the salt solution ln the capillary tube using a
marker.
6 At five-mlnute intervals for a total of 60 minutes, use a ruler to
measure (in millimetres) the distance moved by the solution from the
original mark.
7 Tabulate your results.
8 Plot a graph to show the relationship between the distance moved
by the sa lt solution and the time taken.
2 Fi!J
Questions
I Which structure in the experiment functions as the partially
permeable membrane?
2 Where was the region of higher concentration of water molecules?
3 Where was the region of lower concentration of water molecul es?
4 State the direction of the net movement of the water molecules.
5 Why did the salt solution rise in the capillary tube?
9
·1 · Th e cell
Osmosis in living organisms
Figures 1.13 and 1.14 show the effect that dilute and concent
have on plant and animal cells due to the process of osmosis · rated 5oluu 0lls
.
'0 , .
j'
.
·o
dilutv
..
.
• concentration
the same inside
and outside the
cell
.
• no net movement
of water
:
.
more dilute
external
environment
(more water)
more
external
environment
(more water)
• water moves into cell
• water moves into cell
• water moves out of cell
• cell becomes turgid
(cell wall prevents cell
from bursting)
• cell becomes flaccid
Figure 1.13
The effect of different external environments
on plant cells.
• cell becomes turgid
water moves out of ool
,. cell shrinks
• cell bursts because
it has no cell wall
(plasmolysis)
The effect of diffcrem external environmen1s
on animal cells.
Figure 1.14
How osmosis is used
• Plants take up water from the soil through their roots by the process of
osmosis.
• In food preservation: the process of salting is a very common method of
preserving perishables such as fish . The salt provides a very concentrated
external environment (see figure 1.14 above), which causes the moveweoi
of water out of the cell and the cell shrinks. This process kills bacteria by
dehydrating them.
10
11