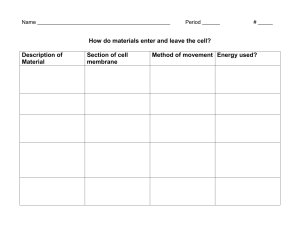
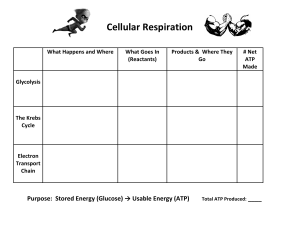
Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis UNIT II CELLS – The Fundamental Unit of Life [Chapter 6] o All organisms are made of cells o The cell is the simplest collection of matter that can live o Cell structure is correlated to cellular function o All cells are related by their descent from earlier cells o Though usually too small to be seen by the unaided eye, cells can be complex 1 Microscopy A. Scientists use microscopes to visualize cells too small to see with the naked eye B. The quality of an image depends on i. Magnification, the ratio of an object’s image size to its real size ii. Resolution, the measure of the clarity of the image, or the minimum distance of two distinguishable points iii. Contrast, visible differences in parts of the sample C. Resolution is inversely related to the wavelength used to visualize (Light, electron) D. Light Microscopes i. In a light microscope (LM), visible light passes through a specimen and then through glass lenses, which magnify the image ii. LMs can magnify effectively to about 1,000 times the size of the actual specimen iii. Various techniques enhance contrast and enable cell components to be stained or labeled iv. Most subcellular structures, including organelles (membrane-enclosed compartments), are too small to be resolved by an LM E. Electron Microscopes i. Two basic types of electron microscopes (EMs) are used to study subcellular structures ii. Scanning electron microscopes (SEMs) focus a beam of electrons onto the surface of a specimen, providing images that look 3-D 1. Surface covered with a film of Gold, beam excites gold really looking at the energy given off by gold iii. Transmission electron microscopes (TEMs) focus a beam of electrons through a specimen 1. TEMs are used mainly to study the internal structure of cells 2. Sample stained with a heavy metal (Urinyl Acetetate) iv. By the way, FSU is one of the world leaders in electron microscopy (TITAN Krios) 2. Cell Fractionation A. Cell fractionation takes cells apart and separates the major organelles from one another B. Ultracentrifuges fractionate cells into their component parts - (Other methods include chromatography, organic extractions, precipitation) C. Cell fractionation enables scientists to determine the functions of organelles 1 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis D. Biochemistry and cytology help correlate cell function with structure 3. There are two types of cells: A. The basic structural and functional unit of every organism is one of two types of cells: prokaryotic or eukaryotic i. Only organisms of the domains Bacteria and Archaea consist of prokaryotic cells ii. Protists, fungi, animals, and plants all consist of eukaryotic cells B. Basic characteristics of all cells (Prokaryotic or Eukaryotic): i. Plasma membrane 1. a selective barrier that allows sufficient passage of oxygen, nutrients, and waste to service the volume of every cell 2. The general structure of a biological membrane is a double layer of phospholipids ii. Cytosol - semifluid interior substance cytosol iii. Chromosomes (carry genes) iv. Ribosomes (make proteins) C. Basic characteristics of Prokaryotic cells i. No nucleus ii. DNA in an unbound region called the nucleoid iii. No membrane-bound organelles iv. Cytoplasm bound by the plasma membrane D. Basic characteristics of Eukaryotic cells i. DNA in a nucleus that is bounded by a membranous nuclear envelope ii. Membrane-bound organelles iii. Cytoplasm in the region between the plasma membrane and nucleus 4. Cell size A. Eukaryotic cells are generally much larger than prokaryotic cells B. The logistics of carrying out cellular metabolism sets limits on the size of cells C. The surface area to volume ratio of a cell is critical D. As the surface area increases by a factor of n2, the volume increases by a factor of n3 E. Small cells have a greater surface area relative to volume 2 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis ORGANELLES Groups a. Endomembrane system i. Nucleus - Information central ii. Endoplasmic Reticulum - Factory iii. Golgi Apparatus - Shipping and Receiving iv. Lysozomes - Digestive Compartments, Disassembly v. Vacuoles - Maintenance compartments vi. [Plasma Membrane] vii. The membranes of this system are either directly connected or connected by transport vesicles b. Energy conversion viii. Mitochondria ix. Chloroplasts x. Peroxisomes 1. The Nucleus 1. The nucleus contains most of the cell’s genes and is usually the most conspicuous organelle 2. The nuclear envelope encloses the nucleus, separating it from the cytoplasm 3. The nuclear membrane is a double membrane; each membrane consists of a lipid bilayer 4. Pores regulate the entry and exit of molecules from the nucleus 5. The shape of the nucleus is maintained by the nuclear lamina, which is composed of protein filaments 6. In the nucleus, DNA and proteins form genetic material called chromatin 7. Chromatin condenses to form discrete chromosomes 8. The nucleolus is located within the nucleus and is the site of ribosomal RNA (rRNA) synthesis 2. Ribosomes - responsible for the conversion of genetic material to protein 1. Ribosomes are particles made of ribosomal RNA and protein 2. Ribosomes carry out protein synthesis in two locations: i. In the cytosol (free ribosomes) - generally make proteins for use in the cytosol ii. On the outside of the endoplasmic reticulum or the nuclear envelope (bound ribosomes) - generally make proteins for transport through a membrane (lysozomes, nucleus, plasma membrane) 3. Endoplamic Reticulum i. The endoplasmic reticulum (ER) accounts for more than half of the total membrane in many eukaryotic cells ii. The ER membrane is continuous with the nuclear envelope iii. There are two distinct regions of ER: 1. Smooth ER, which lacks ribosomes a. Synthesizes lipids b. Metabolizes carbohydrates c. Detoxifies poison d. Stores calcium 3 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 2. Rough ER, with ribosomes studding its surface a. Has bound ribosomes, which secrete glycoproteins (proteins covalently bonded to carbohydrates) b. Distributes transport vesicles, proteins surrounded by membranes c. Is a membrane factory for the cell 4. Golgi Apparatus i. The Golgi apparatus consists of flattened membranous sacs called cisternae ii. Functions of the Golgi apparatus: 1. Modifies products of the ER 2. Manufactures certain macromolecules 3. Sorts and packages materials into transport vesicles 5. Lysozomes i. A lysosome is a membranous sac of hydrolytic enzymes that can digest macromolecules ii. Lysosomal enzymes can hydrolyze 1. proteins 2. fats 3. polysaccharides 4. nucleic acids iii. Some types of cell can engulf another cell by phagocytosis; this forms a food vacuole iv. A lysosome fuses with the food vacuole and digests the molecules v. Lysosomes also use enzymes to recycle the cell’s own organelles and macromolecules, a process called autophagy 6. Vacuoles i. A plant cell or fungal cell may have one or several vacuoles 1. Food vacuoles are formed by phagocytosis 2. Contractile vacuoles, found in many freshwater protists, pump excess water out of cells 3. Central vacuoles, found in many mature plant cells, hold organic compounds and water 4 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis Changing energy from one form to another • Mitochondria are the sites of cellular respiration, a metabolic process that generates ATP • Chloroplasts, found in plants and algae, are the sites of photosynthesis • Peroxisomes are oxidative organelles • Mitochondria and chloroplasts o Are not part of the endomembrane system o Have a double membrane o Have proteins made by free ribosomes o Contain their own DNA 3. Mitochondria i. Are in nearly all eukaryotic cells ii. They have a smooth outer membrane and an inner membrane folded into cristae iii. The inner membrane creates two compartments: intermembrane space and mitochondrial matrix iv. Some metabolic steps of cellular respiration are catalyzed in the mitochondrial matrix v. Cristae present a large surface area for enzymes that synthesize ATP 4. Chloroplasts i. The chloroplast is a member of a family of organelles called plastids (closely related plant organelles) ii. Chloroplasts contain the green pigment chlorophyll, as well as enzymes and other molecules that function in photosynthesis iii. Chloroplasts are found in leaves and other green organs of plants and in algae iv. Chloroplast structure includes: i. Thylakoids, membranous sacs, stacked to form a granum ii. Stroma, the internal fluid 5. Peroxisomes i. Peroxisomes are specialized metabolic compartments bounded by a single membrane ii. Oxygen is used to break down different types of molecules iii. Peroxisomes produce hydrogen peroxide - transfer hydrogens to 02 iv. Convert H2O2 (which is toxic) to water CYTOSKELETON 1. The cytoskeleton is a network of fibers extending throughout the cytoplasm 2. It organizes the cell’s structures and activities, anchoring many organelles 3. It is composed of three types of molecular structures: a. Microtubules - Slide i. the thickest of the three components of the cytoskeleton ii. hollow rods about 25 nm in diameter and about 200 nm to 25 microns long iii. Functions of microtubules: 1. Shaping the cell 2. Guiding movement of organelles 5 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 3. Separating chromosomes during cell division iv. Centrosomes and Centrioles 1. In many cells, microtubules grow out from a centrosome near the nucleus 2. The centrosome is a “microtubule-organizing center” 3. In animal cells, the centrosome has a pair of centrioles, each with nine triplets of microtubules arranged in a ring – slide v. Cilia and Flagella 1. Microtubules control the beating of cilia and flagella, locomotor appendages of some cells 2. Cilia and flagella differ in their beating patterns 3. Cilia and flagella share a common ultrastructure: a. A core of microtubules sheathed by the plasma membrane b. A basal body that anchors the cilium or flagellum c. A motor protein called dynein, which drives the bending movements of a cilium or flagellum 4. Dynein “walking” moves flagella and cilia: a. Dynein arms alternately grab, move, and release the outer microtubules b. Protein cross-links limit sliding c. Forces exerted by dynein arms cause doublets to curve, bending the cilium or flagellum b. Microfilaments, i. also called actin filaments ii. the thinnest components iii. Solid rods about 7 nm in diameter, built as a twisted double chain of actin subunits iv. The structural role of microfilaments is to bear tension, resisting pulling forces within the cell v. They form a 3-D network called the cortex just inside the plasma membrane to help support the cell’s shape vi. Bundles of microfilaments make up the core of microvilli of intestinal cells vii. Microfilaments that function in cellular motility contain the protein myosin in addition to actin 1. In muscle cells, a. thousands of actin filaments are arranged parallel to one another b. Thicker filaments composed of myosin interdigitate with the thinner actin fibers 2. In Amebiod movement a. Pseudopodia (cellular extensions) extend and contract through the reversible assembly and contraction of actin subunits into microfilaments 3. Cytoplasmic streaming a. circular flow of cytoplasm within cells b. This streaming speeds distribution of materials within the cell 6 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis c. In plant cells, actin-myosin interactions and sol-gel transformations drive cytoplasmic streaming c. Intermediate filaments i. fibers with diameters in a middle range ii. Intermediate filaments range in diameter from 8–12 nanometers, larger than microfilaments but smaller than microtubules iii. They support cell shape and fix organelles in place iv. Intermediate filaments are more permanent cytoskeleton fixtures than the other two classes Extracellular components and connections between cells help coordinate cellular activities 1. Most cells synthesize and secrete materials that are external to the plasma membrane 2. These extracellular structures include: a. Cell walls of plants b. The extracellular matrix (ECM) of animal cells c. Intercellular junctions 3. The Cell Wall of plants a. Extracellular structure b. distinguishes plant cells from animal cells c. Prokaryotes, fungi, and some protists also have cell walls d. Protects the plant cell, maintains its shape, and prevents excessive uptake of water e. Made of cellulose fibers embedded in other polysaccharides and protein f. Plant cell walls may have multiple layers: i. Primary cell wall: relatively thin and flexible ii. Middle lamella: thin layer between primary walls of adjacent cells iii. Secondary cell wall (in some cells): added between the plasma membrane and the primary cell wall g. Plasmodesmata are channels between adjacent plant cells 4. The ExtracellularMatrix a. Animal cells lack cell walls but are covered by an elaborate extracellular matrix (ECM) b. Made up of glycoproteins such as collagen, proteoglycans, and fibronectin c. ECM proteins bind to receptor proteins in the plasma membrane called integrins d. Functions of the ECM: i. Support ii. Adhesion iii. Movement iv. Regulation 5. Intercellular Junctions a. Neighboring cells in tissues, organs, or organ systems often adhere, interact, and communicate through direct physical contact b. Intercellular junctions facilitate this contact 7 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis c. Types of intercellular junctions i. Plasmodesmata - channels that perforate plant cell walls. Through plasmodesmata, water and small solutes (and sometimes proteins and RNA) can pass from cell to cell ii. Tight junctions - membranes of neighboring cells are pressed together, preventing leakage of extracellular fluid iii. Desmosomes - (anchoring junctions) fasten cells together into strong sheets iv. Gap junctions - (communicating junctions) provide cytoplasmic channels between adjacent cells 8 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis MEMBRANE STRUCTURE AND FUNCTION [Chapter 7] 1. The Plasma Membrane • The plasma membrane is the boundary that separates the living cell from its surroundings • The plasma membrane exhibits selective permeability, allowing some substances to cross it more easily than others a. Phospholipids i. Phospholipids are the most abundant lipid in the plasma membrane ii. Phospholipids are amphipathic molecules, containing hydrophobic and hydrophilic regions iii. The fluid mosaic model states that a membrane is a fluid structure with a “mosaic” of various proteins embedded in it b. History i. In 1935, Hugh Davson and James Danielli proposed a sandwich model in which the phospholipid bilayer lies between two layers of globular proteins ii. Later studies found problems with this model, particularly the placement of membrane proteins, which have hydrophilic and hydrophobic regions iii. In 1972, J. Singer and G. Nicolson proposed that the membrane is a mosaic of proteins dispersed within the bilayer, with only the hydrophilic regions exposed to water iv. Freeze-fracture studies of the plasma membrane supported the fluid mosaic model v. Freeze-fracture is a specialized preparation technique that splits a membrane along the middle of the phospholipid bilayer c. The Fluidity of Membranes i. Phospholipids in the plasma membrane can move within the bilayer ii. Most of the lipids, and some proteins, drift laterally iii. Rarely does a molecule flip-flop transversely across the membrane iv. As temperatures cool, membranes switch from a fluid state to a solid state v. The temperature at which a membrane solidifies depends on the types of lipids vi. Membranes rich in unsaturated fatty acids are more fluid that those rich in saturated fatty acids vii. Membranes must be fluid to work properly; they are usually about as fluid as salad oil viii. The Role of Cholesterol 1. The steroid cholesterol has different effects on membrane fluidity at different temperatures 2. At warm temperatures (such as 37°C), cholesterol restrains movement of phospholipids 3. At cool temperatures, it maintains fluidity by preventing tight packing d. The Functions of Membrane Proteins i. A membrane is a collage of different proteins embedded in the fluid matrix of the lipid bilayer ii. Proteins determine most of the membrane’s specific functions iii. Peripheral proteins are bound to the surface of the membrane iv. Integral proteins penetrate the hydrophobic core 1. Integral proteins that span the membrane are called transmembrane proteins 9 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 2. The hydrophobic regions of an integral protein consist of one or more stretches of nonpolar amino acids, often coiled into alpha helices v. Six major functions of membrane proteins: 1. Transport 2. Enzymatic activity 3. Signal transduction 4. Cell-cell recognition 5. Intercellular joining 6. Attachment to the cytoskeleton and extracellular matrix (ECM) vi. Cell-Cell Recognition 1. Cells recognize each other by binding to surface molecules, often carbohydrates, on the plasma membrane 2. Membrane carbohydrates may be covalently bonded to lipids (forming glycolipids) or more commonly to proteins (forming glycoproteins) 3. Carbohydrates on the external side of the plasma membrane vary among species, individuals, and even cell types in an individual e. Synthesis and Sidedness of Membranes i. Membranes have distinct inside and outside faces ii. The asymmetrical distribution of proteins, lipids, and associated carbohydrates in the plasma membrane is determined when the membrane is built by the ER and Golgi apparatus f. Membrane Structure Results in Selective Permeability i. Hydrophobic (nonpolar) molecules, such as hydrocarbons, can dissolve in the lipid bilayer and pass through the membrane rapidly ii. Polar molecules, such as sugars, do not cross the membrane easily g. Transport Proteins i. Transport proteins allow passage of hydrophilic substances across the membrane ii. Some transport proteins, called channel proteins, have a hydrophilic channel that certain molecules or ions can use as a tunnel iii. Channel proteins called aquaporins facilitate the passage of water iv. Other transport proteins, called carrier proteins, bind to molecules and change shape to shuttle them across the membrane v. A transport protein is specific for the substance it moves h. Passive Transport i. Diffusion is the tendency for molecules to spread out evenly into the available space ii. Although each molecule moves randomly, diffusion of a population of molecules may exhibit a net movement in one direction iii. At dynamic equilibrium, as many molecules cross one way as cross in the other direction iv. Substances diffuse down their concentration gradient, the difference in concentration of a substance from one area to another v. No work must be done to move substances down the concentration gradient vi. The diffusion of a substance across a biological membrane is passive transport because it requires no energy from the cell to make it happen 10 Biological Science I BSC 2010 i. j. Tue/Thr Dr. Charrel-Dennis Osmosis i. Osmosis is the diffusion of water across a selectively permeable membrane ii. Water diffuses across a membrane from the region of lower solute concentration to the region of higher solute concentration Water Balance i. Cells without Walls 1. Tonicity is the ability of a solution to cause a cell to gain or lose water 2. Isotonic solution: Solute concentration is the same as that inside the cell; no net water movement across the plasma membrane 3. Hypertonic solution: Solute concentration is greater than that inside the cell; cell loses water 4. Hypotonic solution: Solute concentration is less than that inside the cell; cell gains water 5. Hypertonic or hypotonic environments create osmotic problems for organisms 6. Osmoregulation, the control of water balance, is a necessary adaptation for life in such environments 7. The protist Paramecium, which is hypertonic to its pond water environment, has a contractile vacuole that acts as a pump ii. Cells with Walls 1. Cell walls help maintain water balance 2. A plant cell in a hypotonic solution swells until the wall opposes uptake; the cell is now turgid (firm) 3. If a plant cell and its surroundings are isotonic, there is no net movement of water into the cell; the cell becomes flaccid (limp), and the plant may wilt 4. In a hypertonic environment, plant cells lose water; eventually, the membrane pulls away from the wall, a usually lethal effect called plasmolysis iii. Facilitated Diffusion: Passive Transport Aided by Proteins 1. In facilitated diffusion, transport proteins speed the passive movement of molecules across the plasma membrane 2. Channel proteins provide corridors that allow a specific molecule or ion to cross the membrane 3. Channel proteins include a. Aquaporins, for facilitated diffusion of water b. Ion channels that open or close in response to a stimulus (gated channels) 4. Carrier proteins undergo a subtle change in shape that translocates the solute-binding site across the membrane 5. Some diseases are caused by malfunctions in specific transport systems, for example the kidney disease cystinuria iv. Active Transport - uses energy to move solutes against their gradients 1. Facilitated diffusion is still passive because the solute moves down its concentration gradient 2. Some transport proteins, however, can move solutes against their concentration gradients 11 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 3. 4. 5. 6. Active transport moves substances against their concentration gradient Active transport requires energy, usually in the form of ATP Active transport is performed by specific proteins embedded in the membranes Active transport allows cells to maintain concentration gradients that differ from their surroundings 7. The sodium-potassium pump is one type of active transport system 8. How Ion Pumps Maintain Membrane Potential a. Membrane potential is the voltage difference across a membrane b. Voltage is created by differences in the distribution of positive and negative ions c. Two combined forces, collectively called the electrochemical gradient, drive the diffusion of ions across a membrane: i. A chemical force (the ion’s concentration gradient) ii. An electrical force (the effect of the membrane potential on the ion’s movement) iii. An electrogenic pump is a transport protein that generates voltage across a membrane iv. The sodium-potassium pump is the major electrogenic pump of animal cells v. The main electrogenic pump of plants, fungi, and bacteria is a proton pump v. Cotransport: Coupled Transport by a Membrane Protein 1. Cotransport occurs when active transport of a solute indirectly drives transport of another solute 2. Plants commonly use the gradient of hydrogen ions generated by proton pumps to drive active transport of nutrients into the cell vi. Bulk transport across the plasma membrane occurs by exocytosis and endocytosis 1. Small molecules and water enter or leave the cell through the lipid bilayer or by transport proteins 2. Large molecules, such as polysaccharides and proteins, cross the membrane in bulk via vesicles 3. Bulk transport requires energy 4. Exocytosis a. In exocytosis, transport vesicles migrate to the membrane, fuse with it, and release their contents b. Many secretory cells use exocytosis to export their products 5. Endocytosis a. In endocytosis, the cell takes in macromolecules by forming vesicles from the plasma membrane b. Endocytosis is a reversal of exocytosis, involving different proteins c. There are three types of endocytosis: i. Phagocytosis (“cellular eating”) 1. In phagocytosis a cell engulfs a particle in a vacuole 12 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 2. The vacuole fuses with a lysosome to digest the particle ii. Pinocytosis (“cellular drinking”) 1. In pinocytosis, molecules are taken up when extracellular fluid is “gulped” into tiny vesicles iii. Receptor-mediated endocytosis 1. In receptor-mediated endocytosis, binding of ligands to receptors triggers vesicle formation 13 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis AN INTRODUCTION TO METABOLISM [Chapter 8] 1. Energy and life a. The living cell is a miniature chemical factory where thousands of reactions occur b. The cell extracts energy and applies energy to perform work c. Some organisms even convert energy to light, as in bioluminescence 2. An organism’s metabolism transforms matter and energy, subject to the laws of thermodynamics a. Metabolism i. totality of an organism’s chemical reactions ii. an emergent property of life that arises from interactions between molecules within the cell b. A metabolic pathway i. begins with a specific molecule and ends with a product ii. Each step is catalyzed by a specific enzyme c. Catabolic pathways i. release energy by breaking down complex molecules into simpler compounds ii. Cellular respiration, the breakdown of glucose in the presence of oxygen, is an example of a pathway of catabolism d. Anabolic pathways i. consume energy to build complex molecules from simpler ones ii. The synthesis of protein from amino acids is an example of anabolism e. Bioenergetics i. the study of how organisms manage their energy resources ii. Energy is the capacity to cause change iii. Energy exists in various forms, some of which can perform work 1. Kinetic energy is energy associated with motion 2. Heat (thermal energy) is kinetic energy associated with random movement of atoms or molecules 3. Potential energy is energy that matter possesses because of its location or structure 4. Chemical energy is potential energy available for release in a chemical reaction 5. Energy can be converted from one form to another 3. The laws of energy transformation a. Thermodynamics is the study of energy transformations b. A closed system, such as that approximated by liquid in a thermos, is isolated from its surroundings c. An open system, energy and matter can be transferred between the system and its surroundings d. Organisms are open systems e. The First Law of Thermodynamics - the principle of conservation of energy i. The energy of the universe is constant: ii. Energy can be transferred and transformed, but it cannot be created or destroyed f. The Second Law of Thermodynamics 14 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis i. During every energy transfer or transformation, some energy is unusable, and is often lost as heat ii. Every energy transfer or transformation increases the entropy (disorder) of the universe g. Living cells unavoidably convert organized forms of energy to heat h. Spontaneous processes i. occur without energy input ii. they can happen quickly or slowly iii. If no energy input, it must increase the entropy of the universe 4. Biological Order and Disorder a. Cells create ordered structures from less ordered materials b. Organisms replace ordered forms of matter and energy with less ordered forms c. Energy flows into an ecosystem in the form of light and exits in the form of heat d. The evolution of more complex organisms does not violate the second law of thermodynamics e. Entropy (disorder) may decrease in an organism, but the universe’s total entropy increases 5. The free-energy change of a reaction tells us whether or not the reaction occurs spontaneously a. Biologists want to know which reactions occur spontaneously and which require input of energy b. To do so, they need to determine energy changes that occur in chemical reactions c. A living system’s free energy is energy that can do work when temperature and pressure are uniform, as in a living cell d. The change in free energy (∆G) during a process is related to the change in enthalpy, or change in total energy (∆H), change in entropy (∆S), and temperature in Kelvin (T): a. ∆G = ∆H – T∆S e. Only processes with a negative ∆G are spontaneous f. Spontaneous processes can be harnessed to perform work 6. Free Energy, Stability, and Equilibrium a. Free energy is a measure of a system’s instability, its tendency to change to a more stable state i. More free energy (higher G), Less stable, Greater work capacity ii. Less free energy (lower G), More stable, Less work capacity b. During a spontaneous change, free energy decreases and the stability of a system increases i. In a spontaneous change 1. the free energy of the system decreases (∆G < 0) 2. The system becomes more stable 3. The released free energy can be harnessed to do work c. Equilibrium is a state of maximum stability d. A process is spontaneous and can perform work only when it is moving toward equilibrium 7. Exergonic and Endergonic Reactions in Metabolism a. An exergonic reaction proceeds with a net release of free energy and is spontaneous i. In a spontaneous change 15 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 1. the free energy of the system decreases (∆G < 0) 2. The system becomes more stable 3. The released free energy can be harnessed to do work b. An endergonic reaction absorbs free energy from its surroundings and is nonspontaneous i. In a nonspontaneous change 1. the free energy of the system increases (∆G > 0) 2. The system becomes less stable 3. Work is required (energy is required) to create this less stable state 8. Equilibrium and Metabolism a. Reactions in a closed system eventually reach equilibrium and then do no work b. Cells are not in equilibrium; they are open systems experiencing a constant flow of materials c. A defining feature of life is that metabolism is never at equilibrium d. A catabolic pathway in a cell releases free energy in a series of reactions e. Closed and open hydroelectric systems can serve as analogies 9. ATP powers cellular work by coupling exergonic reactions to endergonic reactions a. A cell does three main kinds of work: i. Chemical ii. Transport iii. Mechanical b. To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one c. Most energy coupling in cells is mediated by ATP d. The Structure and Hydrolysis of ATP i. ATP (adenosine triphosphate) is the cell’s energy shuttle ii. ATP is composed of 1. ribose (a sugar) 2. adenine (a nitrogenous base) 3. and three phosphate groups iii. The bonds between the phosphate groups of ATP’s tail can be broken by hydrolysis iv. Energy is released from ATP when the terminal phosphate bond is broken v. This release of energy comes from the chemical change to a state of lower free energy, not from the phosphate bonds themselves 10. How ATP Performs Work a. The three types of cellular work (mechanical, transport, and chemical) are powered by the hydrolysis of ATP b. In the cell, the energy from the exergonic reaction of ATP hydrolysis can be used to drive an endergonic reaction c. Overall, the coupled reactions are exergonic 16 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis d. ATP drives endergonic reactions by phosphorylation, transferring a phosphate group to some other molecule, such as a reactant e. The recipient molecule is now phosphorylated 11. The Regeneration of ATP a. ATP is a renewable resource that is regenerated by addition of a phosphate group to adenosine diphosphate (ADP) b. The energy to phosphorylate ADP comes from catabolic reactions in the cell c. The chemical potential energy temporarily stored in ATP drives most cellular work 12. Enzymes speed up metabolic reactions by lowering energy barriers a. A catalyst is a chemical agent that speeds up a reaction without being consumed by the reaction b. An enzyme is a catalytic protein c. Hydrolysis of sucrose by the enzyme sucrase is an example of an enzyme-catalyzed reaction 13. The Activation Energy Barrier a. Every chemical reaction between molecules involves bond breaking and bond forming b. The initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (EA) c. Activation energy is often supplied in the form of heat from the surroundings d. How Enzymes Lower the EA Barrier i. Enzymes catalyze reactions by lowering the EA barrier ii. Enzymes do not affect the change in free energy (∆G); instead, they hasten reactions that would occur eventually iii. In enzymatic reactions, the substrate binds to the active site of the enzyme = catalytic site iv. The reactant that an enzyme acts on is called the enzyme’s substrate v. The enzyme binds to its substrate, forming an enzyme-substrate complex vi. The active site is the region on the enzyme where the substrate binds vii. Induced fit of a substrate brings chemical groups of the active site into positions that enhance their ability to catalyze the reaction viii. The active site can lower an EA barrier by 1. Orienting substrates correctly 2. Straining substrate bonds 3. Providing a favorable microenvironment 4. Covalently bonding to the substrate 14. Effects of Local Conditions on Enzyme Activity a. An enzyme’s activity can be affected by b. General environmental factors, such as temperature and pH i. Each enzyme has an optimal temperature in which it can function ii. Each enzyme has an optimal pH in which it can function c. Chemicals that specifically influence the enzyme 17 Biological Science I BSC 2010 i. ii. iii. iv. Tue/Thr Dr. Charrel-Dennis Cofactors are nonprotein enzyme helpers Cofactors may be inorganic (such as a metal in ionic form) or organic An organic cofactor is called a coenzyme Coenzymes include vitamins 15. Enzyme Inhibitors a. Competitive inhibitors bind to the active site of an enzyme, competing with the substrate b. Noncompetitive inhibitors bind to another part of an enzyme, causing the enzyme to change shape and making the active site less effective c. Examples of inhibitors include toxins, poisons, pesticides, and antibiotics 16. Regulation of enzyme activity helps control metabolism a. Chemical chaos would result if a cell’s metabolic pathways were not tightly regulated b. Regulation occurs via: i. Switching on or off the genes that encode specific enzymes or by regulating the activity of enzymes ii. Allosteric regulation may either inhibit or stimulate an enzyme’s activity 1. Allosteric regulation occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site 2. Most allosterically regulated enzymes are made from polypeptide subunits 3. Each enzyme has active and inactive forms a. The binding of an activator stabilizes the active form of the enzyme b. The binding of an inhibitor stabilizes the inactive form of the enzyme 4. Cooperativity is a form of allosteric regulation that can amplify enzyme activity a. In cooperativity, binding by a substrate to one active site stabilizes favorable conformational changes at all other subunits 5. Identification of Allosteric Regulators a. Allosteric regulators are attractive drug candidates for enzyme regulation b. Inhibition of proteolytic enzymes called caspases may help management of inappropriate inflammatory responses iii. Feedback Inhibition 1. In feedback inhibition, the end product of a metabolic pathway shuts down the pathway 2. Feedback inhibition prevents a cell from wasting chemical resources by synthesizing more product than is needed 17. Specific Localization of Enzymes Within the Cell a. Structures within the cell help bring order to metabolic pathways b. Some enzymes act as structural components of membranes 18. In eukaryotic cells, some enzymes reside in specific organelles; for example, enzymes for cellular respiration are located in mitochondria 18 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 19 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis CELLULAR RESPIRATION AND FERMENTATION [CHAPTER 9] 1. Overview: Life Is Work • To perform their many tasks, living cells require energy from outside sources. • Energy enters most ecosystems as sunlight and leaves as heat. • In contrast, the chemical elements essential for life are recycled. • Photosynthesis generates oxygen and organic molecules that the mitochondria of eukaryotes (including plants and algae) use as fuel for cellular respiration. • Cells harvest the chemical energy stored in organic molecules and use it to regenerate ATP, the molecule that drives most cellular work. • Respiration has three key pathways: glycolysis, the citric acid cycle, and oxidative phosphorylation. 2. Catabolic pathways yield energy by oxidizing organic fuels. • Catabolic metabolic pathways release the energy stored in complex organic molecules. • Electron transfer plays a major role in these pathways. • The arrangement of atoms of organic molecules represents potential energy. • Enzymes catalyze the systematic degradation of organic molecules that are rich in energy to simpler waste products that have less energy. • Some of the released energy is used to do work; the rest is dissipated as heat. • One type of catabolic process, fermentation, leads to the partial degradation of sugars without the use of oxygen. • A more efficient and widespread catabolic process, aerobic respiration, consumes oxygen as a reactant to complete the breakdown of a variety of organic molecules. • Most eukaryotic and many prokaryotic organisms can carry out aerobic respiration. • Some prokaryotes use compounds other than oxygen as reactants in a similar process called anaerobic respiration. • Although cellular respiration technically includes both aerobic and anaerobic processes, the term is commonly used to refer only to the aerobic process. • Aerobic respiration is similar in broad principle to the combustion of gasoline in an automobile engine after oxygen is mixed with hydrocarbon fuel. • Food is the fuel for respiration. The exhaust is carbon dioxide and water. • The overall catabolic process is: • • organic compounds + O2 à CO2 + H2O + energy (ATP + heat). Carbohydrates, fats, and proteins can all be used as the fuel, but it is most useful to consider glucose: • C6H12O6 + 6O2 à 6CO2 + 6H2O + energy (ATP + heat) • The catabolism of glucose is exergonic, with DG = −686 kcal per mole of glucose. • Some of this energy is used to produce ATP, which can perform cellular work. 20 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 3. Redox reactions release energy when electrons move closer to electronegative atoms. • Catabolic pathways transfer the electrons stored in food molecules, releasing energy that is used to synthesize ATP. • Reactions that result in the transfer of one or more electrons (e−) from one reactant to another are oxidation-reduction reactions, or redox reactions. • The loss of electrons from a substance is called oxidation. • The addition of electrons to another substance is called reduction. • Adding electrons is called reduction because negatively charged electrons added to an atom reduce the amount of positive charge of that atom. • The formation of table salt from sodium and chloride, Na + Cl à Na+ + Cl−, is a redox reaction. • Sodium is oxidized, and chlorine is reduced (its charge drops from 0 to −1). • More generally: Xe− + Y à X + Ye−. • X, the electron donor, is the reducing agent and reduces Y by donating an electron to it. • Y, the electron recipient, is the oxidizing agent and oxidizes X by removing its electron. • Redox reactions require both a donor and an acceptor. • Redox reactions also occur when the transfer of electrons is not complete but involves a change in the degree of electron sharing in covalent bonds. • In the combustion of methane to form water and carbon dioxide, the nonpolar covalent bonds of methane (C—H) and oxygen (O=O) are converted to polar covalent bonds (C=O and O—H). In a few cases, a molecule may have polar bonds, but in a symmetrical arrangement which then gives rise to a nonpolar molecule such as carbon dioxide. - When methane reacts with oxygen to form carbon dioxide, electrons end up farther away from the carbon atom and closer to their new covalent partners, the oxygen atoms, which are very electronegative. - In effect, the carbon atom has partially “lost” its shared electrons. Thus, methane has been oxidized. - When oxygen reacts with the hydrogen from methane to form water, the electrons of the covalent bonds are drawn closer to the oxygen. - In effect, each oxygen atom has partially “gained” electrons, and so the oxygen molecule has been reduced. - Oxygen is very electronegative and is one of the most potent of all oxidizing agents. - Energy must be added to pull an electron away from an atom. - An electron loses potential energy when it shifts from a less electronegative atom toward a more electronegative one. - A redox reaction that relocates electrons closer to oxygen, such as the burning of methane, releases chemical energy that can do work. The two atoms of the oxygen molecule (O2) share their electrons equally. The more electronegative the atom, the more energy is required to take an electron away from it. 21 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 4. Organic fuel molecules are oxidized during cellular respiration. • Respiration, the oxidation of glucose and other molecules in food, is a redox process. • In a series of reactions, glucose is oxidized and oxygen is reduced. • The electrons lose potential energy along the way, and energy is released. • Organic molecules that contain an abundance of hydrogen are excellent fuels. • The bonds of these molecules are a source of “hilltop” electrons, whose energy may be released as the electrons “fall” down an energy gradient when they are transferred to oxygen. • As hydrogen is transferred from glucose to oxygen, the energy state of the electron changes. • In respiration, the oxidation of glucose transfers electrons to a lower energy state, releasing energy that becomes available for ATP synthesis. • The main energy foods, carbohydrates and fats, are reservoirs of electrons associated with hydrogen. • These molecules are stable because of the barrier of activation energy. • Without this barrier, a food molecule like glucose would combine almost instantaneously with O2. • If activation energy is supplied by igniting glucose, it burns in air, releasing 686 kcal (2,870 kJ) of heat per mole of glucose (about 180 g). • This reaction cannot happen at body temperatures. • Instead, enzymes within cells lower the barrier of activation energy, allowing sugar to be oxidized in a series of steps. 5. The “fall” of electrons during respiration is stepwise, via NAD+ and an electron transport chain. • Cellular respiration does not oxidize glucose in a single step that transfers all the hydrogen in the fuel to oxygen at one time. • Rather, glucose and other fuels are broken down in a series of steps, each catalyzed by a specific enzyme. • At key steps, electrons are stripped from the glucose. • In many oxidation reactions, the electron is transferred with a proton, as a hydrogen atom. • The hydrogen atoms are not transferred directly to oxygen but are passed first to a coenzyme called NAD+ (nicotinamide adenine dinucleotide). - “An organic cofactor is called a coenzyme” • As an electron acceptor, NAD+ functions as an oxidizing agent during respiration. • How does NAD+ trap electrons from glucose? • Dehydrogenase enzymes strip two hydrogen atoms from the substrate (glucose), thus oxidizing it. • The enzyme passes two electrons and one proton to NAD+. • The other proton is released as H+ to the surrounding solution. • By receiving two electrons and only one proton, NAD+ has its charge neutralized when it is reduced to NADH. • NAD+ functions as the oxidizing agent in many of the redox steps during the breakdown of glucose. • The electrons carried by NADH lose very little of their potential energy in this process. 22 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis • Each NADH molecule formed during respiration represents stored energy. This energy is tapped to synthesize ATP as electrons “fall” down an energy gradient from NADH to oxygen. • How are electrons extracted from glucose and stored in NADH finally transferred to oxygen? • Unlike the explosive release of heat energy that occurs when H2 and O2 are combined (with a spark for activation energy), cellular respiration uses an electron transport chain to break the fall of electrons to O2 into several steps. • The electron transport chain consists of several molecules (primarily proteins) built into the inner membrane of a mitochondrion of eukaryotic cells and the plasma membrane of aerobically respiring prokaryotes. • Electrons released from food are shuttled by NADH to the “top” higher-energy end of the chain. • At the “bottom” lower-energy end, oxygen captures the electrons along with H+ to form water. • Electron transfer from NADH to oxygen is an exergonic reaction with a free-energy change of −53 kcal/mol. • Electrons are passed to increasingly electronegative molecules in the chain until they reduce oxygen, the most electronegative receptor. • Each “downhill” carrier is more electronegative than, and thus capable of oxidizing, its “uphill” neighbor, with oxygen at the bottom of the chain. • The electrons removed from glucose by NAD+ fall down an energy gradient in the electron transport chain to a far more stable location in the electronegative oxygen atom. • In summary, during cellular respiration, most electrons travel the following “downhill” route: glucose à NADH à electron transport chain à oxygen. 6. The stages of cellular respiration: a preview. • Respiration occurs in three metabolic stages: glycolysis, the citric acid cycle, and the electron transport chain and oxidative phosphorylation. • Glycolysis occurs in the cytosol. It begins catabolism by breaking glucose into two molecules of pyruvate. • The citric acid cycle occurs in the mitochondrial matrix of eukaryotic cells or in the cytoplasm of prokaryotes. It completes the breakdown of glucose by oxidizing a derivative of pyruvate to carbon dioxide. • Several steps in glycolysis and the citric acid cycle are redox reactions in which dehydrogenase enzymes transfer electrons from substrates to NAD+, forming NADH. • In the third stage of respiration, the electron transport chain accepts electrons from the breakdown products of the first two stages (most often via NADH). • In the electron transport chain, the electrons move from molecule to molecule until they combine with molecular oxygen and hydrogen ions to form water. • As the electrons are passed along the chain, the energy released at each step in the chain is stored in a form the mitochondrion (or prokaryotic cell) can use to make ATP. • This mode of ATP synthesis is called oxidative phosphorylation because it is powered by the redox reactions of the electron transport chain. • In eukaryotic cells, the inner membrane of the mitochondrion is the site of electron transport and chemiosmosis, the processes that together constitute oxidative phosphorylation. 23 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis • In prokaryotes, these processes take place in the plasma membrane. • Oxidative phosphorylation produces almost 90% of the ATP generated by respiration. • Some ATP is also formed directly during glycolysis and the citric acid cycle by substrate-level phosphorylation, in which an enzyme transfers a phosphate group from an organic substrate molecule to ADP, forming ATP. • For each molecule of glucose degraded to carbon dioxide and water by respiration, the cell makes as many as 32 ATP, each with 7.3 kcal/mol of free energy. • Respiration uses the small steps in the respiratory pathway to break the large denomination of energy contained in glucose into the small change of ATP. • The quantity of energy in ATP is more appropriate for the energy level of work required in the cell. Glycolysis harvests chemical energy by oxidizing glucose to pyruvate. • During glycolysis, glucose, a six-carbon sugar, is split into two three-carbon sugars. • These smaller sugars are then oxidized and rearranged to form two molecules of pyruvate, the ionized form of pyruvic acid. • Each of the ten steps in glycolysis is catalyzed by a specific enzyme. • These steps can be divided into two phases. 1. In the energy investment phase, the cell spends ATP. 2. In the energy payoff phase, this investment is repaid with interest. ATP is produced by substrate-level phosphorylation, and NAD+ is reduced to NADH by electrons released by the oxidation of glucose. • The net yield from glycolysis is 2 ATP and 2 NADH per glucose. o No CO2 is produced during glycolysis. • Glycolysis can occur whether or not O2 is present. • If O2 is present, the chemical energy stored in pyruvate and NADH can be extracted by the citric acid cycle and oxidative phosphorylation. The citric acid cycle completes the energy-yielding oxidation of organic molecules. • More than three-quarters of the original energy in glucose is still present in the two molecules of pyruvate. • If molecular oxygen is present in eukaryotic cells, pyruvate enters the mitochondrion, where enzymes of the citric acid cycle complete the oxidation of the organic fuel to carbon dioxide. o In prokaryotic cells, this process occurs in the cytoplasm. • After pyruvate enters the mitochondrion via active transport, it is converted to a compound called acetyl coenzyme A, or acetyl CoA. • This step, the junction between glycolysis and the citric acid cycle, is accomplished by a multienzyme complex that catalyzes three reactions. 24 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis 1. A carboxyl group is removed as CO2. The carbon dioxide is fully oxidized and thus has little chemical energy. 2. The remaining two-carbon fragment is oxidized to form acetate. An enzyme transfers the pair of electrons to NAD+ to form NADH. 3. Acetate combines with coenzyme A to form the very reactive molecule acetyl CoA. • Due to the chemical nature of the CoA group, a sulfur-containing compound derived from a B vitamin, acetyl CoA has a high potential energy. o In other words, the reaction of acetyl CoA to yield lower-energy products is highly exergonic. • Acetyl CoA is now ready to feed its acetyl group into the citric acid cycle for further oxidation. • The citric acid cycle is also called the tricarboxylic acid cycle or the Krebs cycle. o The latter name honors Hans Krebs, who was largely responsible for elucidating the cycle’s pathways in the 1930s. • The citric acid cycle oxidizes organic fuel derived from pyruvate. • Three CO2 molecules are released, including the one released during the conversion of pyruvate to acetyl CoA. • The cycle generates one ATP per turn by substrate-level phosphorylation. • Most of the chemical energy is transferred to NAD+ and a related electron carrier, the coenzyme FAD, during the redox reactions. • The reduced coenzymes, NADH and FADH2, transfer high-energy electrons to the electron transport chain. • The citric acid cycle has eight steps, each catalyzed by a specific enzyme. o The acetyl group of acetyl CoA joins the cycle by combining with the compound oxaloacetate, forming citrate. o The next seven steps decompose the citrate back to oxaloacetate. o It is the regeneration of oxaloacetate that makes this process a cycle. • For each acetyl group that enters the cycle, 3 NAD+ are reduced to NADH. • In one step, electrons are transferred to FAD instead of NAD+. • Then FAD accepts 2 electrons and 2 protons to become FADH2. • In the cells of plants, bacteria, and a few animal tissues, the citric acid cycle forms an ATP molecule by substrate-level phosphorylation. • In most animal tissue cells, guanosine triphosphate (GTP) is formed by the same process of substrate-level phosphorylation. • GTP may be used to synthesize an ATP or to directly power work in the cell. • The output from this step is the only ATP generated directly by the citric acid cycle. 25 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis • Most of the ATP produced by respiration results from oxidative phosphorylation, as the NADH and FADH2 produced by the citric acid cycle relay the electrons extracted from food to the electron transport chain. • This process supplies the necessary energy for the phosphorylation of ADP to ATP. During oxidative phosphorylation, chemiosmosis couples electron transport to ATP synthesis. • Only 4 of 32 ATP ultimately produced by the respiration of glucose are produced by substrate-level phosphorylation. o Two ATP are produced during glycolysis, and 2 ATP are produced during the citric acid cycle. • NADH and FADH2 account for most of the energy extracted from glucose. • These reduced coenzymes link glycolysis and the citric acid cycle to oxidative phosphorylation, which uses energy released by the electron transport chain to power ATP synthesis. The inner mitochondrial membrane couples electron transport to ATP synthesis. • The electron transport chain is a collection of molecules embedded in the cristae, the folded inner membrane of the mitochondrion. o In prokaryotes, the electron transport chain is located in the plasma membrane. • The folding of the inner membrane to form cristae increases its surface area, providing space for thousands of copies of the chain in each mitochondrion. • Most components of the chain are proteins that exist in multiprotein complexes numbered I– IV. • Tightly bound to these proteins are prosthetic groups, nonprotein components essential for catalysis. • Electrons drop in free energy as they pass down the electron transport chain. • During electron transport along the chain, electron carriers alternate between reduced and oxidized states as they accept and donate electrons. o Each component of the chain becomes reduced when it accepts electrons from its “uphill” neighbor, which is less electronegative. o It then returns to its oxidized form as it passes electrons to its more electronegative “downhill” neighbor. • Electrons carried by NADH are transferred to the first molecule in the electron transport chain, a flavoprotein. • In the next redox reaction, the flavoprotein returns to its oxidized form as it passes electrons to an iron-sulfur protein. • The iron-sulfur protein then passes the electrons to a compound called ubiquinone, a small hydrophobic molecule and the only member of the electron transport chain that is not a protein. • Most of the remaining electron carriers between ubiquinone and oxygen are proteins called cytochromes. o The prosthetic group of each cytochrome is a heme group with an iron atom that accepts and donates electrons. • The last cytochrome of the chain, cyt a3, passes its electrons to oxygen, which is very electronegative. 26 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis • Each oxygen atom also picks up a pair of hydrogen ions from the aqueous solution to form water. • The electrons carried by FADH2 have lower free energy and are added at a lower energy level than those carried by NADH. o The electron transport chain provides about one-third less energy for ATP synthesis when the electron donor is FADH2 rather than NADH. • The electron transport chain generates no ATP directly. • Its function is to break the large free-energy drop from food to oxygen into a series of smaller steps that release energy in manageable amounts. Chemiosmosis couples electron transport and energy release to ATP synthesis. • A protein complex in the cristae, ATP synthase, actually makes ATP from ADP and Pi. • ATP synthase works like an ion pump running in reverse. o Ion pumps usually use ATP as an energy source to transport ions against their gradients. o Enzymes can catalyze a reaction in either direction, depending on the DG for the reaction, which is affected by the local concentrations of reactants and products. o Rather than hydrolyzing ATP to pump protons against their concentration gradient, under the conditions of cellular respiration, ATP synthase uses the energy of an existing ion gradient to power ATP synthesis. • The power source for the ATP synthase is a difference in the concentrations of H+ on opposite sides of the inner mitochondrial membrane. o This is also a pH gradient. • This process, in which energy stored in the form of a hydrogen ion gradient across a membrane is used to drive cellular work such as the synthesis of ATP, is called chemiosmosis. o Here, osmosis refers to the flow of H+ across a membrane. • From studying the structure of ATP synthase, scientists have learned how the flow of H+ through this large enzyme powers ATP generation. • ATP synthase is a multisubunit complex with four main parts, each made up of multiple polypeptides. • Protons move one by one into binding sites on one of the parts (the rotor), causing it to spin in a way that catalyzes ATP production from ADP and inorganic phosphate. o ATP synthase is the smallest molecular rotary motor known in nature. • Part of the complex actually spins around in the membrane when the reaction proceeds in the direction of ATP hydrolysis. • Biochemists assumed that the same rotational mechanism was responsible for ATP synthesis, but they lacked experimental evidence. • In 2004, nanotechnology techniques (involving control of matter on the molecular scale) were used to demonstrate that the direction of rotation of one part of the complex in relation to another is solely responsible for either ATP synthesis or ATP hydrolysis by this enzyme. 27 Biological Science I BSC 2010 • Tue/Thr Dr. Charrel-Dennis How does the inner mitochondrial membrane or the prokaryotic plasma membrane generate and maintain the H+ gradient that drives ATP synthesis in the ATP synthase protein complex? o Establishing the H+ gradient is the function of the electron transport chain. o The chain is an energy converter that uses the exergonic flow of electrons to pump H+ across the membrane from the mitochondrial matrix into the intermembrane space. o The H+ has a tendency to diffuse down its gradient. • The ATP synthase molecules are the only place where H+ can diffuse back to the matrix. • The exergonic flow of H+ is used by the enzyme to generate ATP. • This coupling of the redox reactions of the electron transport chain to ATP synthesis is an example of chemiosmosis. • How does the electron transport chain pump protons? o Certain members of the electron transport chain accept and release H+ along with electrons. o At certain steps along the chain, electron transfers cause H+ to be taken up and released into the surrounding solution. o The electron carriers are spatially arranged in the membrane in such a way that protons are accepted from the mitochondrial matrix and deposited in the intermembrane space. o The H+ gradient that results is the proton-motive force, a gradient with the capacity to do work. o The force drives H+ back across the membrane through the specific H+ channels provided by ATP synthases. • Chemiosmosis is an energy-coupling mechanism that uses energy stored in the form of an H+ gradient across a membrane to drive cellular work. • In mitochondria, the energy for proton gradient formation comes from exergonic redox reactions, and ATP synthesis is the work performed. • Chemiosmosis in chloroplasts also generates ATP, but light drives the electron flow down an electron transport chain and H+ gradient formation. • Prokaryotes generate H+ gradients across their plasma membrane. Prokaryotes use the proton-motive force not only to generate ATP but also to pump nutrients and waste products across the membrane and to rotate their flagella. Fermentation and anaerobic cellular respiration: enable cells to produce ATP without the use of oxygen § Most cellular respiration depends on electronegative oxygen to pull electrons down the transport chain § Without oxygen, the electron transport chain will cease to operate § In that case, glycolysis couples with anaerobic respiration or fermentation to produce ATP § Anaerobic respiration uses an electron transport chain with a final electron acceptor other than oxygen, for example, sulfate § Fermentation uses substrate-level phosphorylation instead of an electron transport chain to generate ATP Type of Fermentation 28 Biological Science I BSC 2010 § § § § § § Tue/Thr Dr. Charrel-Dennis Fermentation consists of glycolysis plus reactions that regenerate NAD+, which can be reused by glycolysis Two common types are alcohol fermentation and lactic acid fermentation In alcohol fermentation, pyruvate is converted to ethanol in two steps § The first step releases CO2 from pyruvate § The second step produces NAD+ and ethanol In lactic acid fermentation, pyruvate is reduced by NADH, forming NAD+ and lactate as end products, with no release of CO2 The processes have different mechanisms for oxidizing NADH to NAD+: § In fermentation, an organic molecule (such as pyruvate or acetaldehyde) acts as a final electron acceptor § In cellular respiration, electrons are transferred to the electron transport chain Cellular respiration produces 32 ATP per glucose molecule; fermentation produces 2 ATP per glucose molecule Gycolysis and the citric acid cycle are major intersections to various catabolic and anabolic pathways 29 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis PHOTOSYNTHESIS [CHAPTER 10] 1. The Process That Feeds the Biosphere a. Photosynthesis is the process that converts solar energy into chemical energy b. Directly or indirectly, photosynthesis nourishes almost the entire living world c. Can classify life forms into two groups: d. Autotrophs i. Sustain themselves without eating anything derived from other organisms ii. The producers of the biosphere, iii. Produce organic molecules from CO2 and other inorganic molecules iv. Almost all plants are photoautotrophs, using the energy of sunlight to make organic molecules from H2O and CO2 v. Photosynthesis occurs in plants, algae, certain other protists, and some prokaryotes vi. These organisms feed not only themselves but also most of the living world e. Heterotrophs i. Obtain their organic material from other organisms ii. Consumers of the biosphere iii. Depend on photoautotrophs for food and O2 2. Photosynthesis converts light energy to the chemical energy of food. a. Chloroplasts are structurally similar to and likely evolved from photosynthetic bacteria b. The structural organization of these cells allows for the chemical reactions of photosynthesis c. Leaves are the major locations of photosynthesis d. Their green color is from chlorophyll, the green pigment within chloroplasts e. Light energy absorbed by chlorophyll drives the synthesis of organic molecules in the chloroplast f. CO2 enters and O2 exits the leaf through microscopic pores called stomata g. Chloroplasts are found mainly in cells of the mesophyll, the interior tissue of the leaf h. A typical mesophyll cell has 30–40 chloroplasts i. The chlorophyll is in the membranes of thylakoids (connected sacs in the chloroplast); thylakoids may be stacked in columns called grana j. Chloroplasts also contain stroma, a dense fluid k. Veins deliver water from the roots and carry off sugar from mesophyll cells to nonphotosynthetic areas of the plant. l. Photosynthesis can be summarized as the following equation: 1. 6 CO2 + 12 H2O + Light energy à C6H12O6 + 6 O2 + 6 H2O 3. Splitting of water a. Chloroplasts split H2O into hydrogen and oxygen, incorporating the electrons of hydrogen into sugar molecules 4. Photosynthesis is a redox process a. H2O is oxidized and CO2 is reduced 5. Photosynthesis consists of two parts: 30 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis a. The light reactions (the photo part) and Calvin cycle (the synthesis part) i. The light reactions (in the thylakoids): 1. Split H2O 2. Reduce NADP+ to NADPH 3. Generate ATP from ADP by photophosphorylation ii. The Calvin cycle (in the stroma) 1. Forms sugar from CO2, using ATP and NADPH 2. Begins with carbon fixation, incorporating CO2 into organic molecules 6. The light reactions a. The light reactions convert solar energy to the chemical energy of ATP and NADPH b. Light is a form of electromagnetic energy, also called electromagnetic radiation c. Like other electromagnetic energy, light travels in rhythmic waves d. Wavelength is the distance between crests of waves e. Wavelength determines the type of electromagnetic energy f. The electromagnetic spectrum is the entire range of electromagnetic energy, or radiation g. Visible light consists of wavelengths (including those that drive photosynthesis) that produce colors we can see h. Light also behaves as though it consists of discrete particles, called photons 7. Photosynthetic pigments - the light receptors a. Pigments are substances that absorb visible light b. Different pigments absorb different wavelengths c. Wavelengths that are not absorbed are reflected or transmitted d. Leaves appear green because chlorophyll reflects and transmits green light e. A spectrophotometer measures a pigment’s ability to absorb various wavelengths f. This machine sends light through pigments and measures the fraction of light transmitted at each wavelength g. An absorption spectrum is a graph plotting a pigment’s light absorption versus wavelength h. The absorption spectrum of chlorophyll a suggests that violet-blue and red light work best for photosynthesis i. An action spectrum profiles the relative effectiveness of different wavelengths of radiation in driving a process 8. Excitation of Pigments (Chlorophyll) by light a. When a pigment absorbs light (energy), it goes from a ground state to an excited state, which is unstable b. When excited electrons fall back to the ground state, photons are given off, an afterglow called fluorescence c. If illuminated, an isolated solution of chlorophyll will fluoresce, giving off light and heat 9. A Photosystem: A Reaction-Center Complex Associated with Light-Harvesting Complexes 31 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis a. A photosystem consists of a reaction-center complex (a type of protein complex) surrounded by lightharvesting complexes b. The light-harvesting complexes (pigment molecules bound to proteins) funnel the energy of photons to the reaction center c. A primary electron acceptor in the reaction center accepts an excited electron from chlorophyll a d. Solar-powered transfer of an electron from a chlorophyll a molecule to the primary electron acceptor is the first step of the light reactions e. There are two types of photosystems in the thylakoid membrane i. Photosystem II (PS II) 1. Functions first (the numbers reflect order of discovery) and is best at absorbing a wavelength of 680 nm 2. The reaction-center chlorophyll a of PS II is called P680 ii. Photosystem I 1. Best at absorbing a wavelength of 700 nm 2. The reaction-center chlorophyll a of PS I is called P700 10. Two types of electron Flow in the light cycle: cyclic and linear a. Linear electron flow i. the primary pathway, involves both photosystems and produces ATP and NADPH using light energy ii. A photon hits a pigment and its energy is passed among pigment molecules until it excites P680 iii. An excited electron from P680 is transferred to the primary electron acceptor iv. P680+ (P680 that is missing an electron) is a very strong oxidizing agent v. H2O is split by enzymes, and the electrons are transferred from the hydrogen atoms to P680+, thus reducing it to P680 vi. O2 is released as a by-product of this reaction vii. Each electron “falls” down an electron transport chain from the primary electron acceptor of PS II to PS I viii. Energy released by the fall drives the creation of a proton gradient across the thylakoid membrane ix. Diffusion of H+ (protons) across the membrane drives ATP synthesis x. In PS I (like PS II), transferred light energy excites P700, which loses an electron to an electron acceptor xi. P700+ (P700 that is missing an electron) accepts an electron passed down from PS II via the electron transport chain xii. Each electron “falls” down an electron transport chain from the primary electron acceptor of PS I to the protein ferredoxin (Fd) b. Cyclic Electron Flow i. Cyclic electron flow uses only photosystem I and produces ATP, but not NADPH ii. Cyclic electron flow generates surplus ATP, satisfying the higher demand in the Calvin cycle iii. Some organisms such as purple sulfur bacteria have PS I but not PS II 32 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis iv. Cyclic electron flow is thought to have evolved before linear electron flow v. Cyclic electron flow may protect cells from light-induced damage 11. The Calvin cycle uses ATP and NADPH to convert CO2 to sugar a. The Calvin cycle, like the citric acid cycle, regenerates its starting material after molecules enter and leave the cycle b. The cycle builds sugar from smaller molecules by using ATP and the reducing power of electrons carried by NADPH c. Carbon enters the cycle as CO2 and leaves as a sugar named glyceraldehyde-3-phosphate (G3P) d. For net synthesis of 1 G3P, the cycle must take place three times, fixing 3 molecules of CO2 e. The Calvin cycle has three phases: i. Carbon fixation (catalyzed by rubisco) ii. Reduction iii. Regeneration of the CO2 acceptor (RuBP) RUBISCO CAN BIND O2 WITH CATLYTIC EFFICIENCY 12. Alternative mechanisms of carbon fixation have evolved in hot, arid climates a. Dehydration is a problem for plants, sometimes requiring trade-offs with other metabolic processes, especially photosynthesis b. On hot, dry days, plants close stomata, which conserves H2O but also limits photosynthesis c. The closing of stomata reduces access to CO2 and causes O2 to build up d. These conditions favor a seemingly wasteful process called photorespiration i. In most plants (C3 plants), initial fixation of CO2, via rubisco, forms a three-carbon compound ii. In photorespiration, rubisco adds O2 instead of CO2 in the Calvin cycle iii. Photorespiration consumes O2 and organic fuel and releases CO2 without producing ATP or sugar iv. Photorespiration may be an evolutionary relic because rubisco first evolved at a time when the atmosphere had far less O2 and more CO2 v. Photorespiration limits damaging products of light reactions that build up in the absence of the Calvin cycle vi. In many plants, photorespiration is a problem because on a hot, dry day it can drain as much as 50% of the carbon fixed by the Calvin cycle 13. C4 Plants a. C4 plants minimize the cost of photorespiration by incorporating CO2 into four-carbon compounds in mesophyll cells b. This step requires the enzyme PEP carboxylase c. PEP carboxylase has a higher affinity for CO2 than rubisco does; it can fix CO2 even when CO2 concentrations are low 33 Biological Science I BSC 2010 Tue/Thr Dr. Charrel-Dennis d. These four-carbon compounds are exported to bundle-sheath cells, where they release CO2 that is then used in the Calvin cycle 14. CAM Plants a. Some plants, including succulents, use crassulacean acid metabolism (CAM) to fix carbon b. CAM plants open their stomata at night, incorporating CO2 into organic acids c. Stomata close during the day, and CO2 is released from organic acids and used in the Calvin cycle 34